EnviroMail 08 Canada
Identifying Hazardous Chemicals Required During Field Sampling
Workplace Hazardous Materials Information System (WHMIS) is Canada’s national hazard communication standard. The key elements of the system are hazard classification, cautionary labelling of containers, the provision of (material) safety data sheets ((M)SDSs) and worker education and training programs.

The basis for hazard classification and communication in WHMIS is changing. With the incorporation of the Globally Harmonized System of Classification and Labelling for chemicals (GHS) in WHMIS, the hazard classification and communication requirements of WHMIS have been aligned with those used in the United States and other Canadian trading partners. WHMIS is in a period of transition between two hazard communication regimes - WHMIS 1988 and WHMIS 2015 (which incorporates the GHS).
You will be noticing some changes to sample container labels and preservative vial labels in Canada as ALS conforms with the WHMIS 2015 requirements. ALS will be introducing some colour to your labels. The reasons for these changes are simple:
- To be fully compliant and proactive regarding the upcoming GHS labelling requirements - all suppliers and distributors have until November 30, 2018 to implement the new requirements.
- To provide more visual cues to the hazardous/non-hazardous nature of the chemicals used during sampling and therefore provide a safer workplace environment.
ALS will also be promoting the use of pre-charged containers (labeled in accordance with GHS requirements) as another HSE initiative. Pre-charging or dosing of the sample containers means there is no need to transfer hazardous chemicals (such as nitric acid or sodium hydroxide) in the field, and reduces the waste of the additional plastic preservative vial.
In some cases, such as the preservation of metals and mercury, ALS will be promoting the removal of acids entirely in the field. The regulatory methods used in Canada allow metals and mercury to be preserved upon receipt in the laboratory. For metals, samples must be preserved within 14 days of sample collection to be compliant with the 180 days hold time. For mercury, samples have to preserved within 28 days of sample collection to be compliant with the 28 day hold time.
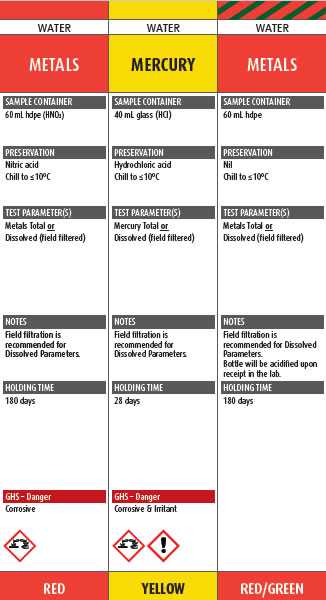
ALS will be introducing a new colour coded and GHS compliant Canadian Sample Collection Pocket Guide to assist with identifiying the labels and container requirements for tests in Canada. The pocket guide will show which test parameters can be tested from each container, the container size and preservative added, useful notes to assist with sampling, holding times for each test, as well as the GHS “signal word” and pictogram.
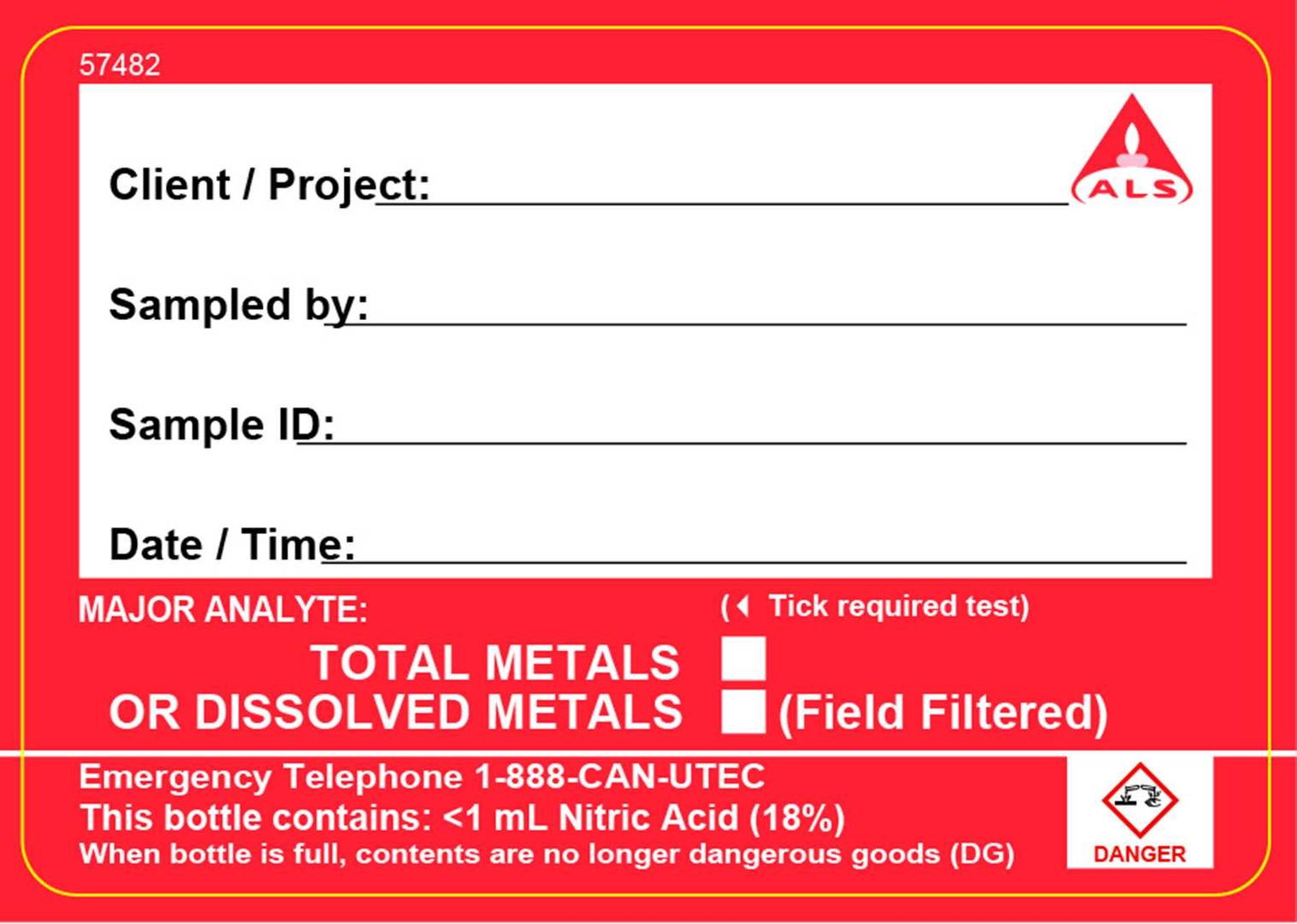
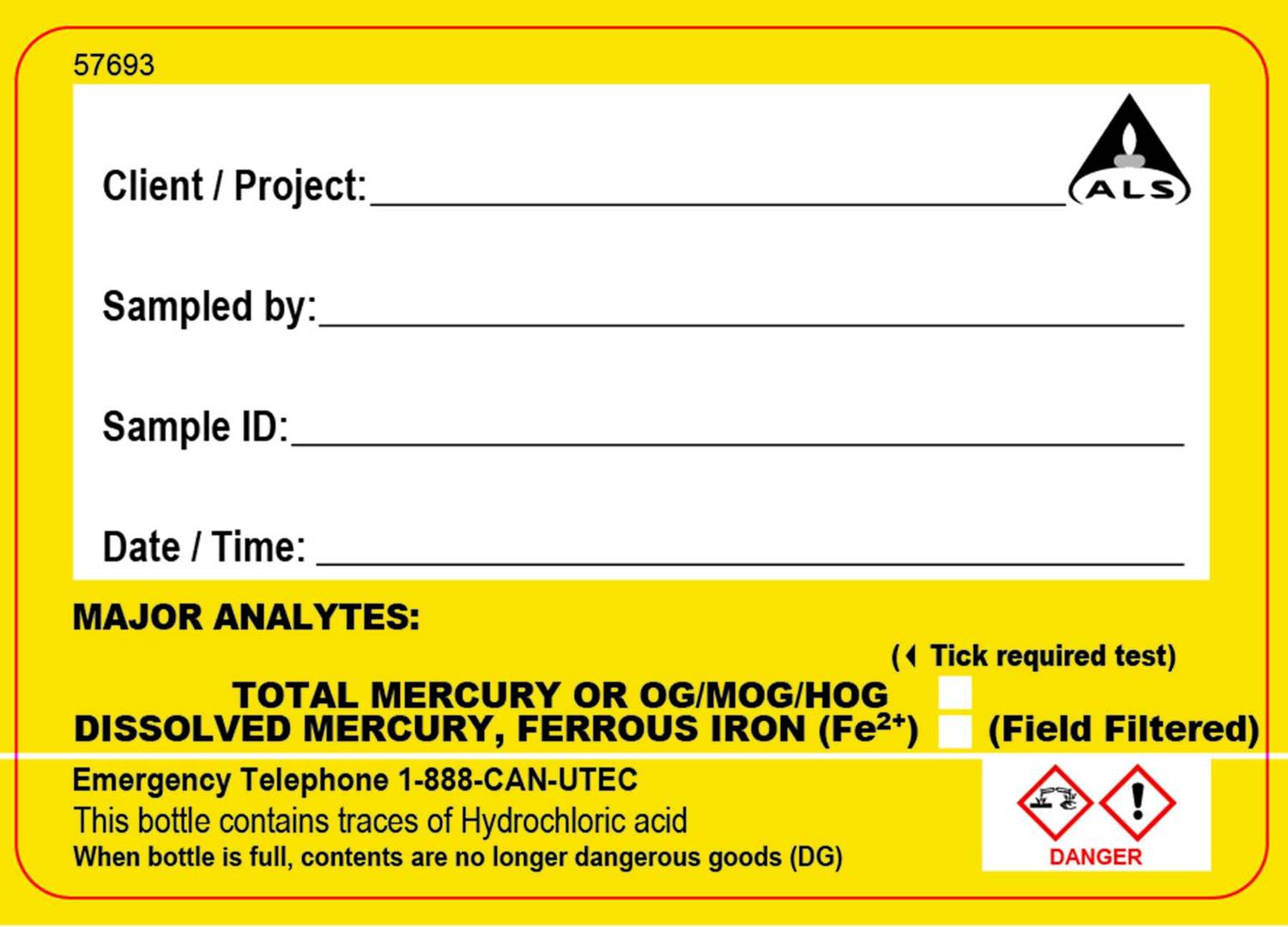
In the excerpt from the guide below, the colour of the preservation - red for nitric acid, yellow for hydrochloric acid, and red/green for acidifed upon receipt at the laboratory are clearly indicated and match the colour of the label attached to the container (shown on the right hand side).
If the container is pre-charged with preservative, the label will include the preservative name, a pictogram within a red diamond to indicate the type of hazard (e.g. carcinogen, irritant, corrosive, etc), a red signal word displayed (e.g. DANGER, WARNING, ETC) and the contact number to call if large volumes of chemical are spilled into the environment, inhaled or splashed on skin or eyes.
ALS always recommends the use of personal protective equipment when dealing with chemicals in the field, which should at a minimum include protective eye wear and chemically resistant gloves. In most first aid situations, rinsing of eyes or skin with copius amounts of water will be adequate first aid. Eyewash bottles are recommended as part of any emergency first aid kit, especially in remote areas, for this reason.
View a complete guide to the 2018 GHS pictograms.