EnviroMail 71 Canada
Ultrashort PFAS: Occurrence, Remediation Challenges, Emerging Regulations, & Analysis
Ultrashort chain per- and polyfluoroalkyl substances (USC-PFAS) are PFAS with three or fewer carbon atoms, and are characterized by their high water solubility, low affinity for organic matter, and high environmental mobility. These properties contribute to their widespread global presence in environmental waters and wastewaters – including drinking waters.
Fig 1. Waste refrigerants can be a source of USC-PFAS

A recent publication found that three USC-PFAS (TFA, PFPrA, and TFMS) were ubiquitous in German drinking waters, and constituted ~98% of the sum of all detected PFAS species.1 Environmental concerns associated with USC-PFAS include their persistence, potential for groundwater contamination, and challenges in remediation due to their resistance to conventional treatment methods.
Occurrence, Uses, and Sources
Ultrashort chain PFAS have been much less studied than their longer chain counterparts, and understanding of their intended and unintended transformation processes is limited. Sources of USC-PFAS include degradation of precursor compounds, atmospheric degradation of hydrofluorocarbon (HFC) and hydrochlorofluorocarbon (HCFC) refrigerants, and intentional direct use in products like batteries. They are also byproducts from historical electrochemical fluorination (ECF) manufacture of longer chain PFAS, and are found in urban and industrial waste, and in aqueous film-forming foams (AFFFs) used in firefighting.
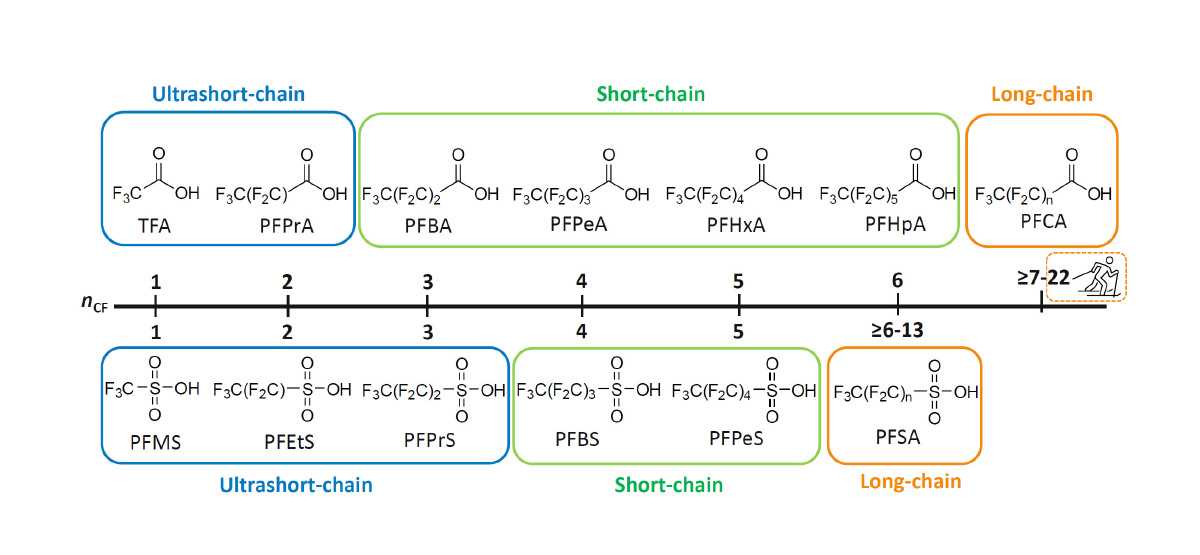
Fig 2. PFAS categorization based on chain length (image courtesy of Phenomenex & SCIEX)
Fate, Transport, and Remediation Challenges
Carbon chain length plays a key role in determining the physicochemical characteristics of PFAS chemicals. Long chain PFAS are more hydrophobic, which makes them amenable to water treatment techniques using adsorption by carbonaceous materials like granular activated carbon (GAC). Short and ultrashort chain PFAS are more hydrophilic and water soluble, which makes them less adsorptive to organic carbon. These attributes mean that USC-PFAS disperse more freely in aqueous environments and are less prone to sorption onto soils, sediments, and other natural solids. The outcome is that USC-PFAS are highly mobile in aquatic environments, and can travel far from contaminant source areas. These same properties make wastewater or groundwater treatment strategies such as GAC and ion exchange much more challenging for ultrashort chain PFAS versus longer chain PFAS such as PFOS or PFOA.
Numerous recent publications have shown detections of ultrashort PFAS compounds (particularly TFA and PFPrA) in surface waters, groundwaters, and drinking waters, confirming their widespread presence in the global environment today.2 TFA in particular is by far the most environmentally abundant PFAS, and is even found consistently in rain (averaging ~ 100-500 ng/L), with concentrations increasing steadily after HCFC and HFC refrigerants replaced CFCs in the 1990s. Some modern hydrofluoroolefin (HFO) refrigerants also degrade to TFA.3,4

Fig 3. Traditional wastewater treatments are less effective for USC-PFAS
Global Regulatory Status
Awareness about the persistence, bioaccumulative nature, and suspected toxicity of C8-C14 PFAS began to attract widespread concern near the turn of the millennium. PFOS was added to the persistent organic pollutants (POPs) list of the Stockholm Convention in 2009, with PFOA and PFHxS added later in 2019 and 2022. With phaseouts of long chain PFAS, manufacturers began to produce short and ultrashort chain alternatives. However, with the change from C8 to C4 and shorter fluorinated carbon chains came a sacrifice to lower technical performance, resulting in the production of greater quantities to achieve equal performance. While it is generally accepted that short and ultrashort chain PFAS have lower toxicological profiles, they are not devoid of environmental risks; their continued use and release in combination with higher loadings, greater mobility, and more challenging remediation suggest that adverse risks may lie ahead.
Although global regulations for ultrashort PFAS are in early stages of development, there are already several European regulations and guidelines for TFA in drinking water, which has been widely detected in tap waters and bottled waters across Europe and in Canada, the USA, and China.2,6 Germany has classified TFA as a reproductive toxin, and introduced a health-based guidance value for TFA in drinking waters of 60 μg/L, with a precautionary goal of < 10 μg/L. Denmark has established a regulatory limit for TFA in drinking water at 9 μg/L, and the Dutch Institute for Public Health and the Environment (RIVM 2023) set a lower indicative drinking water value at 2.2 μg/L.
As with other regions in the Americas, Europe, and Asia-Pacific, Health Canada has committed to monitoring PFAS as a class, and although they have not specifically targeted ultrashort PFAS, they recognize that analytical methods measuring larger lists of PFAS are evolving, and that targeted lists for analysis may expand over time.
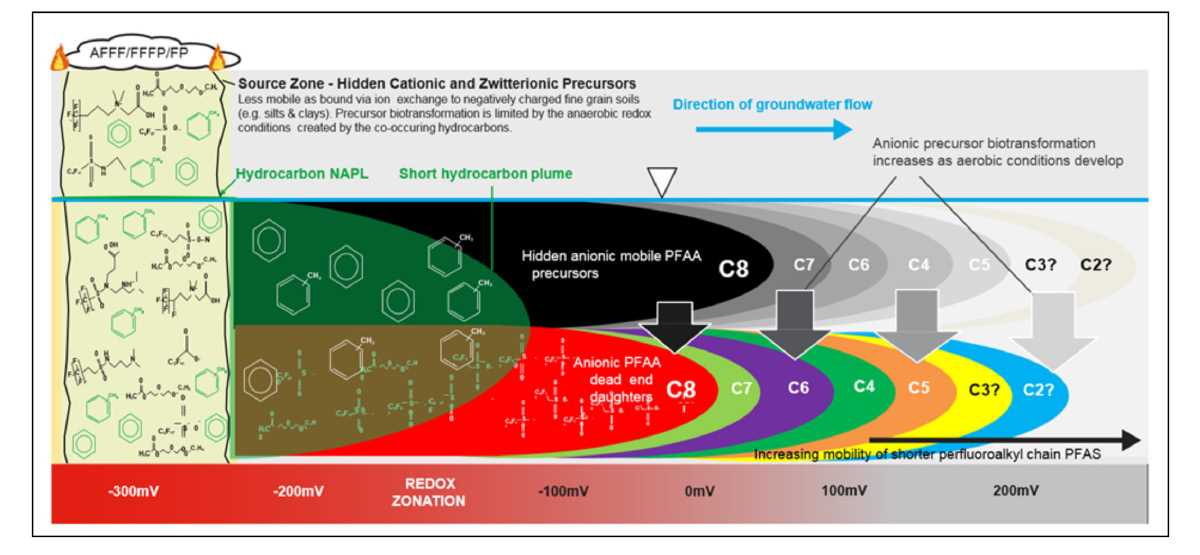
Fig 4. Conceptual site model for PFASs from a fire training area source zone, Arcadis Feb 2019.2 Used with permission.
Testing for USC-PFAS
ALS offers testing of waters for nine ultrashort PFAS through our specialty PFAS laboratory in Sydney, Australia, with the parameter list and Limits of Reporting (LORs) shown in Table 1. Samples are collected in 20 mL HDPE containers without preservation. Suitable containers must be obtained from ALS due to the ubiquitous nature of some USC-PFAS such as TFA. In the absence of well-documented or regulatory holding time criteria, ALS recommends a conservative holding time of 14 days after sample collection, although preliminary in-house stability trials indicate that these analytes can be stable for up to several months after sampling when stored at 4⁰C.
Table 1: ALS Parameter List for USC-PFAS
Table 2: Test & Sampling Details

References
1 Neuwald, I. J. et al. (2022). Ultra-Short-Chain PFASs in the Sources of German Drinking Water: Prevalent, Overlooked, Difficult to Remove, and Unregulated. Environmental Science & Technology, 56 (10), 6380-6390. https://doi.org/10.1021/acs.est.1c07949
2 Ross, I., & Hurst, J. (2019). Managing Risks and Liabilities associated with Per- and Polyfluoroalkyl Substances (PFASs). CL:AIRE Technical Bulletin TB19. CL:AIRE, London, UK.
3 Albers, C. N. & Sültenfuss, J. (2024). A 60-Year Increase in the Ultrashort-Chain PFAS Trifluoroacetate and Its Suitability as a Tracer for Groundwater Age. Environmental Science & Technology Letters, 11 (10), 1090–1095. https://doi.org/10.1021/acs.estlett.4c00525
4 Arp, H. P. H. et al. (2024). The Global Threat from the Irreversible Accumulation of Trifluoroacetic Acid (TFA). Environmental Science & Technology, 58(45), 19925-19935. https://doi.org/10.1021/acs.est.4c06189
5 Ghorbani Gorji, S. et al. (2024). Occurrence of Ultrashort-Chain PFASs in Australian Environmental Water Samples. Environmental Science and Technology Letters, 11 (12), 1362–1369. https://doi.org/10.1021/acs.estlett.4c00750
6 Crini, G., et al. (2025). Assessment of trifluoroacetic acid in tap water from Besançon (France) and bottled water from France, Italy, and Romania. Discover Water 5, 86. https://doi.org/10.1007/s43832-025-00294-y
Please contact your ALS Project Manager for more information about this test service. To ensure samples can be analyzed within the recommended hold time, it is important to pre-arrange sampling and drop-off dates to facilitate prompt shipment of samples to our USC-PFAS testing laboratory.






















































