Enviromail 23 USA - PFAS Analysis by TOP Assay
Perfluoroalkyl Substances (PFAS) are a family of fluorine containing chemicals used in a variety of products including nonstick cookware, waterproof and stain resistant clothing, aqueous film forming foams (AFFF) used in firefighting as well as certain manufacturing processes. Due to their persistence, toxicity and bioaccumulative potential, these compounds are of increased concern to environment and health agencies.

PFAS and Total Oxidizable Precursor Assay
Accredited laboratories currently analyze for approximately 40 PFAS compounds.
Total Oxidizable Precursor Assay is an analytical method with an oxidation step that converts unknown PFAS called precursors to commonly analyzed perfluoroalkyl acids known as terminal PFAS compounds.
After the TOP assay oxidation and analytical process, the increase in measurable PFAS relative to pre-oxidation levels is an estimate of the concentration of PFAS precursors in the sample.
Affected Products
Over several years ALS has performed numerous oxidation tests on aqueous film forming foams (AFFF) in order to obtain a deeper knowledge of the Total Oxidizable Precursor Assay. This includes the impact of concentration plus the differences between first generation and modern foams.
A number of foam products were subject to the TOP alkaline persulfate digest and analyzed by LC/MS/MS. Three products were examined: 3M Light Water™, Ansulite™, and Aer-o-Water™. The first of these products was the classic pre-2000 AFFF while the other two represent more recent short-chain foams. Note that only the Aer-O-Water™ product was in its original packaging while the other products had been supplied as subsamples of uncertain origin.
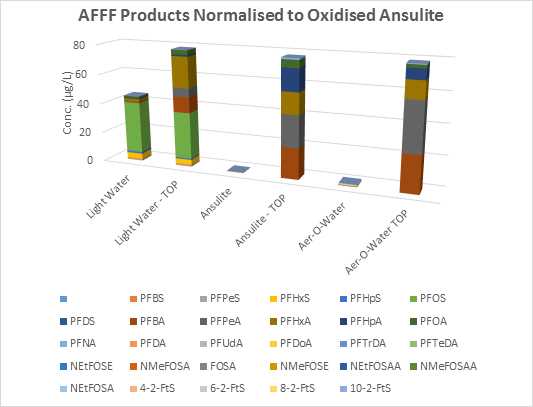
Figure 1 below indicates a significant increase in total PFAS following oxidation for Ansulite™ and Aer-O-Water™. An increase of perfluorocarboxylic acids in 3M Light Water™ may be attributable to unspecified “fluoroalkyl amide derivatives” described in a 1996 MSDS from 3M in Australia. The outcomes of these digests are consistent with results reported in the literature on this subject.
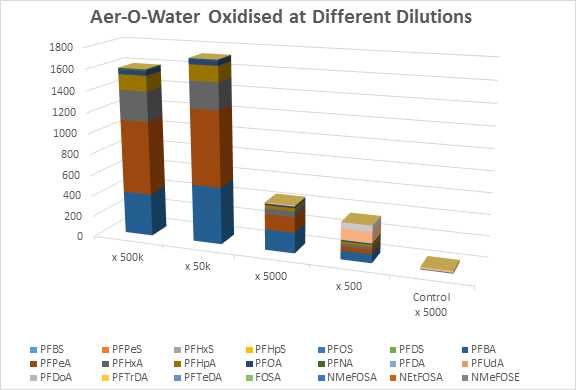
In figure 2, the concentration of the AFFF can affect both qualitative and quantitative outcome of the oxidation process. Oxidation at high dilutions leads to full conversion of the material to carboxylic acids. At higher concentrations, this conversion is less than complete which indicated exhaustion of the oxidant. At a 500-fold dilution, 6:2-FTS is a large component of the composition which is consistent with the presence of fluorotelomer sulfonamido betaines in some modern-day products.
Conclusion
The TOP assay is capable of revealing the presence of PFAS that may, given time, weather to perfluorinated alkyl substances of concern, but is definitely not a predictor of the endpoint of abiotic and biotic breakdown in the field. Oxidation has been well considered as a treatment option. This includes both alkaline and heat activated persulfate, both of which are used in the TOP assay.
To some extent, this may explain the small loss of PFOS observed with oxidation of the 3M Light Water™. In experiments performed at ALS, a 13C-labeled PFOS surrogate was added pre-oxidation and regularly recovered around 80%. Oxidation of a full analytical standard (not under standard conditions) also yielded less than a mass balance when summed, which indicated some loss to shorter chain PFAS carboxylates not normally quantified.
If the oxidant is exhausted either by competition from non-PFAS organic carbon or high concentrations of PFAS, both qualitative and quantitative conversion of AFFF PFAS precursors may be incomplete.
When comparing data between laboratories, it is essential that the oxidation conditions are absolutely standardized and that sample dilutions are reasonably equivalent.
In conclusion, the TOP assay is a useful tool in exposing the potential for ongoing contamination by PFAS compounds through biotic and abiotic weathering processes. Results, however, should be treated with caution, especially where health or ecological risk assessment is required. There may also be a case to expand analytical suites to cover other PFAS that may arise from weathering that might include some oxidation and hydrolysis, and, ideally, to have better models for predicting environmental endpoints of AFFF degradation.
Please contact our PFAS representatives for more details, or to request sampling supplies.
| West |
|
Howard Boorse |
| East |
|
Angela Karst |
| Central |
| Kristin Neir kristin.neir@alsglobal.com 281 530 565 |





















































