EnviroMail 151 Australia
Expanding the scope of PFAS analysis in soils and waters
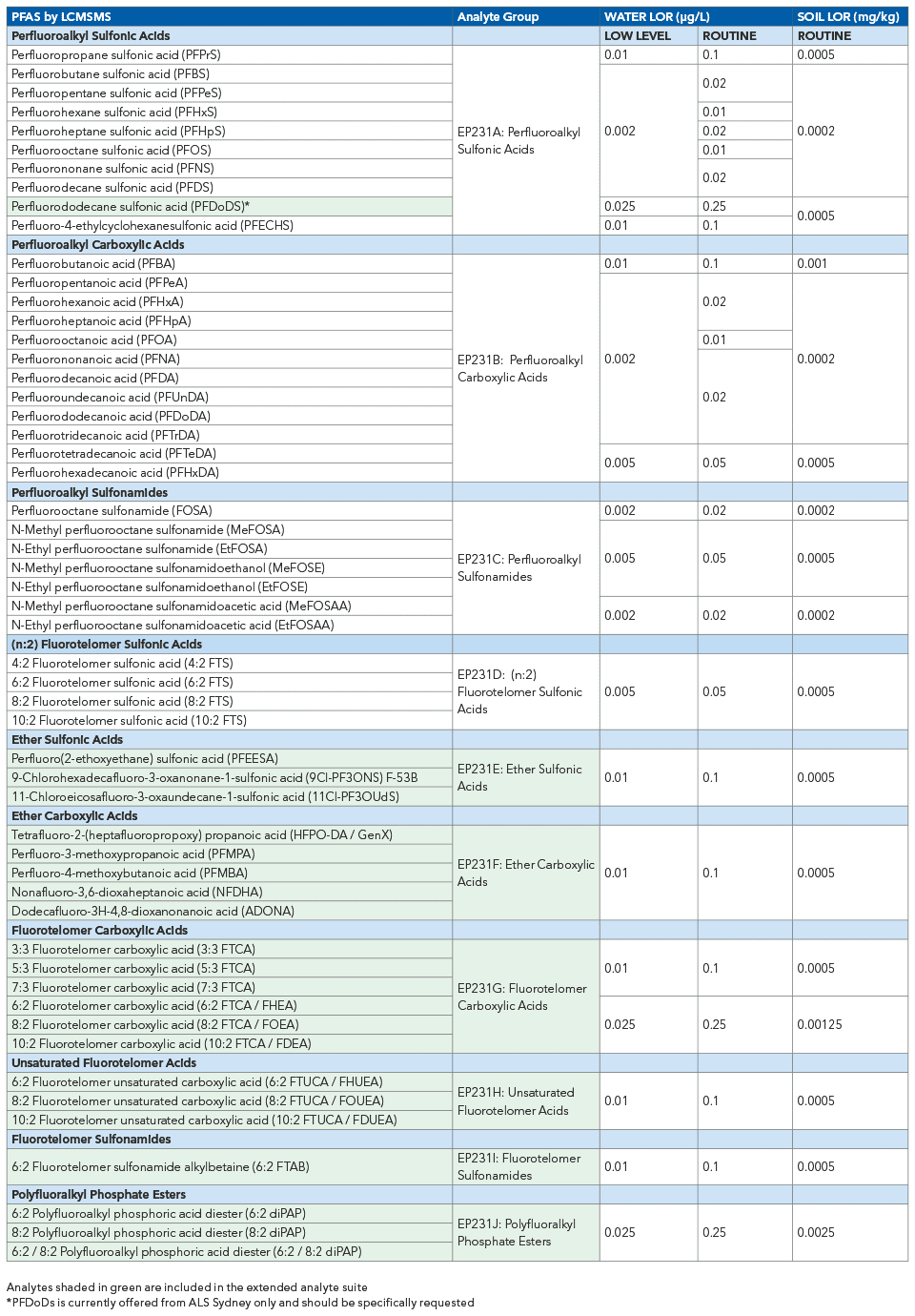
ALS in Australia has now extended its service offering to include 54 PFAS compounds.

The sheer diversity in chemical and physical characteristics of PFAS confounds a ‘one size fits all’ approach to PFAS analytical chemistry. The 54 PFAS suite includes PFAS compounds of varying properties, occurrence, and risk profile, providing broad utility to environmental consultants and specialists. Importantly, all analytes included in US EPA method 1633 are included in ALS Australia’s new service offering.
ALS 54 PFAS Suite
The ALS routine 28-compound list comprised four compound groups:
- Perfluoroalkyl carboxylic acids (PFCAs)
- Perfluoroalkyl sulfonic acids (PFSAs)
- Perfluoroalkyl sulfonamides (FASAs)
- Fluorotelomer sulfonic acids (FTSAs).
With the emergence of new classes of PFAS compounds such as the GenX compound HFPO-DA, an onus on environmental specialists to assess the extent of contamination, and laboratories to develop corresponding methodologies emerged.
Novel PFAS compounds
Six new PFAS compound groups are now available, consisting of 25 new analytes.
The newly added analytes comprise:
- Ether sulfonic acids
- Ether carboxylic acids
- Fluorotelomer carboxylic acids
- Unsaturated fluorotelomer acids
- Fluorotelomer sulfonamides
- Polyfluoralkyl phosphate esters
Furthermore, PFPrS and PFNS are now incorporated into ALS’s routine compound suite growing the longstanding 28-analyte list to a total of 30 routinely reported analytes.
Properties and occurrence of compounds in ALS 54 PFAS suite
Some industrial applications of the newly offered compounds are discussed briefly below to highlight their context in environmental monitoring and site remediation.
Per and poly fluoro ether carboxylic acids (PFECAs) and sulfonic acids (PFESAs)
The PFECAs and PFESAs include both per and poly fluorinated PFAS. Dupont (Chemours) produced tetrafluoro-2-(heptafluoropropoxy) propanoic acid (HFPO-DA), while 3M (Dyneon™) produced Dodecafluoro-3H-4,8-dioxanonanoic acid (ADONA). Both products were used as surfactants and polymerisation aids following phase out of PFOA.
HFPO-DA is perfluorinated and has emerged as a recalcitrant environmental contaminant. It is not susceptible to oxidation under the TOP assay thus being an endpoint compound under normal TOP assay conditions. Conversely ADONA is polyfluorinated with a hydrogen for fluorine substitution adjacent to the ether oxygen. The substitution creates a point in the molecule more readily susceptible to degradation.
Under TOP assay conditions short chain fluorinated compounds are formed which are not accounted for by typical liquid chromatography methods. Both compounds are not accounted for by typical TOP assay methods.
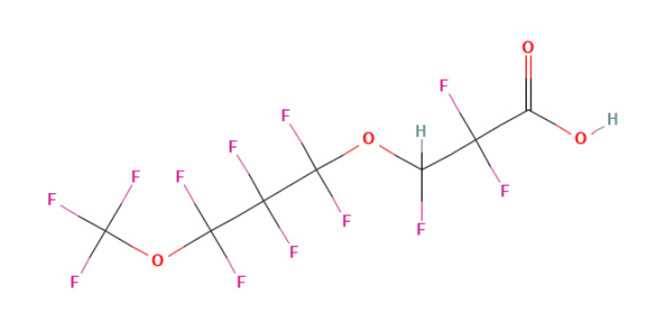
Figure 1a - ADONA is polyfluorinated
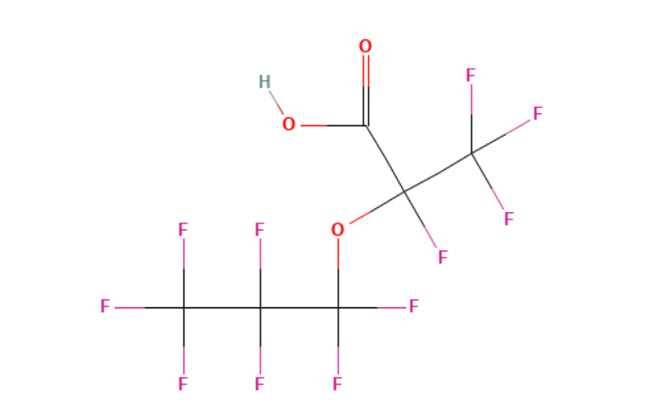
Figure 1b - HFPO-DA is perfluorinated
Chlorinated polyfluorinated ether sulfonates (Cl-PFAESs) were used extensively in China to replace PFOS in metal plating applications. An example is F-53B which contained 6:2 Cl-PFAES (~70%) and the 8:2 homologue as a byproduct (~30%).
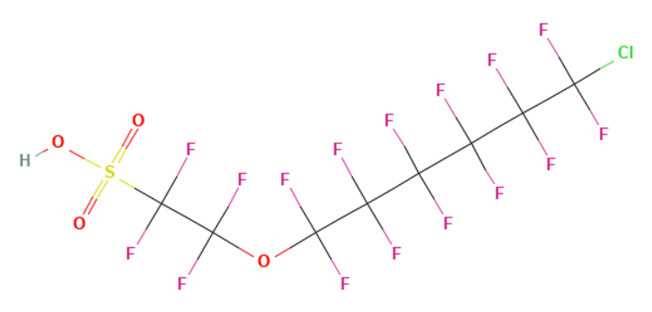
Figure 2a - 9-Chlorohexadecafluoro-3-oxanonane-1-sulfonic acid (9Cl-PF3ONS), or 6:2 Cl-PFAES
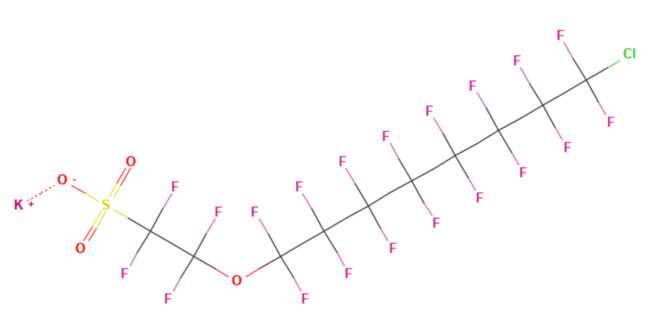
Figure 2b - 11-Chloroeicosafluoro-3-oxaundecane-1-sulfonic acid (11Cl-PF3OUdS), or 8:2 Cl-PFAES
Production of 6:2 Cl-PFAES has been largely associated with metal plating, although this PFAS compound has been detected in Arctic wildlife, and USA soils, suggesting long-range transport and worldwide contamination (Chen 2017). Recent studies indicate detection in a wide variety of environmental media and widespread human exposure in China.
The remaining PFECA and PFESA compounds included in the ALS 54 compound suite—PFEESA, PFMBA, PFMPA, NFDHA—were detected in runoff from a fire event at factory storing chemical and industrial waste in 2019, along with other PFAS compounds including HFPO-DA, 6:2 FTAB, and several polyfluoroalkyl diesters (DiPAPs).
Saturated and unsaturated fluorotelomer carboxylic acids (FTCAs and FTUCAs)
The ALS 54 PFAS suite includes a total of nine FTCA and FTUCA compounds. The significance of this class is their presence in biotransformation and degradation pathways of fluorotelomer alcohols (FTOHs) to PFCAs. FTOHs are suggested to degrade quickly and then follow a degradation pathway that includes, in sequence: fluorotelomer aldehydes (FTALs), even numbered FTCAs, even numbered FTUCAs (among others), odd numbered FTUCAs (among others), odd numbered FTCAs.1 FTCAs and FTUCAs pose a toxicological risk themselves with FTCAs reported to elicit a greater risk than FTUCAs. Hence an understanding of the relative amounts of these compounds provides insight into the age of contamination plumes.
Furthermore, 5:3 FTCA forms as a product of precursor transformation in model landfill reactors, and its presence can be used as an indicator of landfill leachate contamination.
Polyfluoroalkyl diesters (DiPAPs)
DiPAPs have been produced since the 1970s for use in cosmetics, cleaning agents, paper, and food packaging materials. Market demand for analysis of phosphate ester PFAS compounds has increased in recent times, especially from municipal water authorities. DiPAPs have been widely detected in various matrices including biota, wastewater, soil, sediments, biosolids, human serum, and indoor dust.
DiPAPs have been identified as major constituents of PFAS in biosolids based on samples collected from 19 Australian wastewater treatment plants (WWTPs) (Moodie et al, 2018). As a class of compounds, little is known about the environmental fate and occurrence of DiPAPs, compared to, e.g., PFCAs and PFSAs.
Fluorotelomer sulfonamides
6:2 FTAB is perhaps the cornerstone compound in a homologous series of zwitterionic, substituted fluorotelomer based products. The compound has gained attraction mostly in the context of AFFF contamination due to its inclusion in Forafac®, an AFFF that replaced 3M’s Lightwater at various fire training facilities in the early 2000s following phase out of legacy PFAS.
Analytically, 6:2 FTAB poses certain challenges, especially in solid matrices. Prevailing sample preparation methods for routine PFAS lists exhibited variable recovery of 6:2 FTAB suggesting that it binds strongly to solid or particulate matter. While existing techniques recovered 6:2 FTAB adequately in various soils, approximately one in three soil submatrices exhibited nil or very low recoveries. As a result, ALS undertook extensive trials to optimise extraction protocols to demonstrate confident recoveries of 6:2 FTAB across all commonly encountered soils.
PFAS—a never ending story
As interest and demand for analysis of PFAS compounds evolves, ALS will continue to update and validate new procedures to meet market requirements.
ALS laboratories in Brisbane, Sydney, Melbourne and Perth have capabilities to analyse the extended 54 PFAS suite.2 Sampling protocols and holding times remain unchanged, and services are available for standard, low level, and ultra trace limits of reporting.
2 PFDoDs is currently only available via ALS Sydney and should be specificaly requested.
Get in touch with us
Should your project require analysis of any compounds included in the 54 PFAS suite, don’t hesitate to discuss requirements with your project manager. To request the 54 PFAS suite, ALS package EP231X-ALL (standard LORs) or EP231X-LL-ALL (low level) should be noted on CoCs and analysis request documents.
Contact us at one of our leading environmental services laboratories on the details below.
Brisbane
Sydney
Melbourne
Perth
Download as a PDF





















































