EnviroMail 148 Australia - Interpreting TOP Assay
How to avoid common pitfalls

Total Oxidisable Precursor Assay
The likelihood that PFAS not included in routine analytical suites, are lurking in the environment is widely acknowledged. After all, it is cited that >12,000 unique PFAS chemicals have been registered for industrial use. The total oxidisable precursor assay (TOP-A) can assist in removing the veil to this potential PFAS ‘dark matter’, revealing the presence of what have commonly become be known as PFAS ‘precursors’.
The basic principle of TOP-A is easy to understand. Chemical reagents and gentle heating causes formation of hydroxyl radicals, which attack and transform the more complex PFAS precursors into stable perfluorocarboxylic acids (PFCAs). The C4-C16 PFCAs are readily measurable and routinely reported in analytical suites.
Equation 1: Formation of hydroxyl radicals under controlled conditions drives the TOP-A reaction.
S2O82- + [heat] > 2SO4 -. (Sulfate radical)
SO4 -. + OH- > SO42- + OH. (Hydroxyl radical)
Despite the utility of TOP-A, care needs to be taken with data interpretation for several reasons:
Indirect transformation products: Transformation products may not be species with equivalent fluorinated carbon numbers to their precursors. Formation products with less than four carbons, and even mineralisation of fluorine may occur.
Oxidative transformation conditions: Degradation pathways may be different to environmentally relevant ones.
Method standardisation: Different laboratories commonly apply different oxidant strengths. Adjusting experimental conditions on a case-by-case basis canalso be necessary where there is evidence of incomplete oxidation, typically the case where matrix components like high dissolved organic carbon (DOC) show competing oxygen demand.
Incomplete recoveries and transformation to species not included in routine suites: TOP-A is not usually an effective means of assessing fluorine mass balance (eg. compared with the total organic fluorine assay).
Assumptions that TOP-A can account for all the hidden PFAS: Some commercial products, and novel and next generation PFAS are not amenable to oxidative transformation.
TOP-A treatment for a routine and extended analyte list
To elucidate the behaviour of a selection of PFAS compounds under TOP-A and hence assist with data interpretation, ALS subjected single component spikes to TOP-A conditions and monitored recoveries and transformation products to 60 unique PFAS compounds. Small volumes of individual stock standards were spiked, and solvent evaporated in tubes before reconstituting in water for TOP-A. As expected, perfluorinated carboxylic acids were the main end products and mass balance was not seen for any of the converted precursors by summing recoveries of PFAS included in the experiment. The summed molar recoveries of the test PFAS are summarised in the table below.
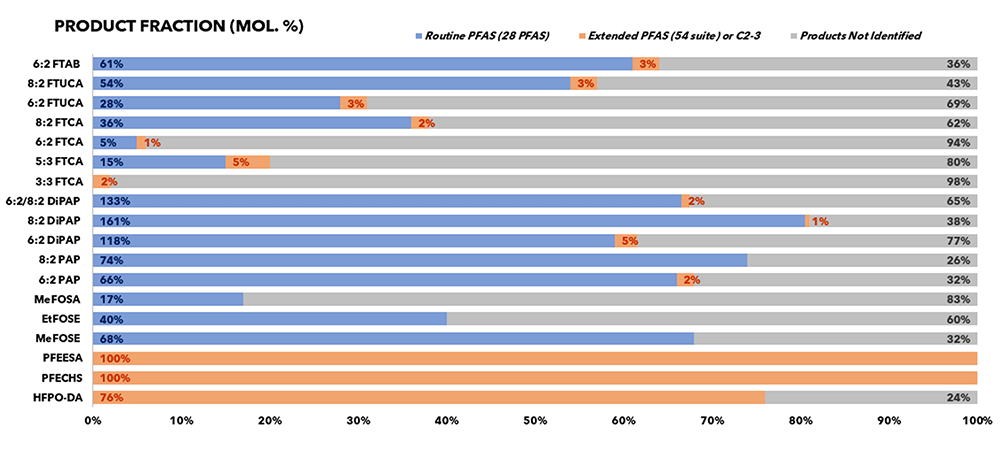
Figure 1: Percent molar yields of a selection of PFAS
*Note that DiPAPs are disubstituted and expected to yield two mole equivalents of reaction products
Specific TOP-A degradation patterning
Specific PFCAs and other products detected post oxidation are summarised by class in Figures 2-5 and recoveries have been converted to molar equivalents to enable easy recovery comparisons between target PFAS and degradation products.Interestingly, only a few short chain products were observed consisting of low levels of TFA and PFPrA from DiPAPs, GenX (HFPO-DA) and 6:2 FTAB. Other experimenters have reported higher formation rates of ultra-short chain PFAS, but also report incomplete conversions. Where discrepancies exist between recoveries reported here and elsewhere attribution may be to the empirical and non-standardised nature of the TOP-A method, and the increased oxidant doses required to effectively convert known precursors.
Fluorotelomer PFAS
Products manufactured via fluorotelomerisation consistently exhibit a suite of PFCA degradation products under TOP-A conditions. TOP-A behaviour of classes of compounds containing the -(CH2)n- moiety characteristic to fluorotelemers are summarised in Figures 2, 3 and (for figure 4, see 6:2 FTAB).
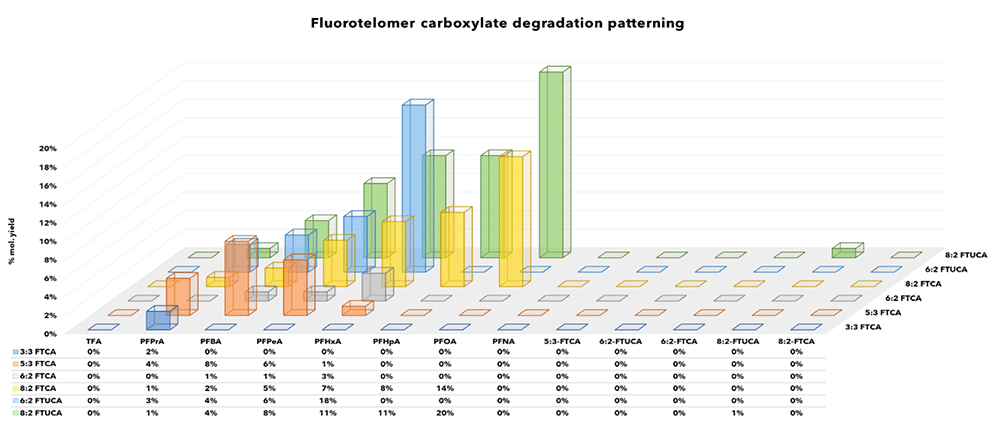
Figure 2: FTCA and FTUCA products
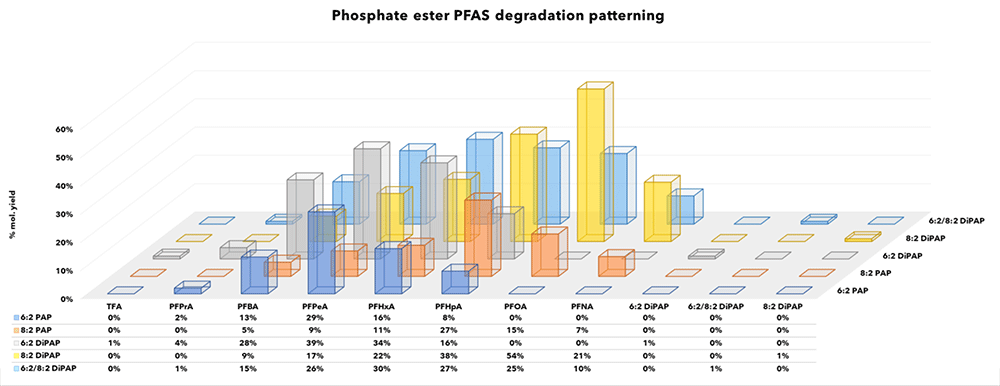
Figure 3: Mono- and Di- phosphate ester products
Electrochemical fluorination products (ECF)
PFAS manufactured by ECF, such as sulfonamide linked products almost always show oxidation products dominated by PFAS of corresponding perfluorinated carbon content.
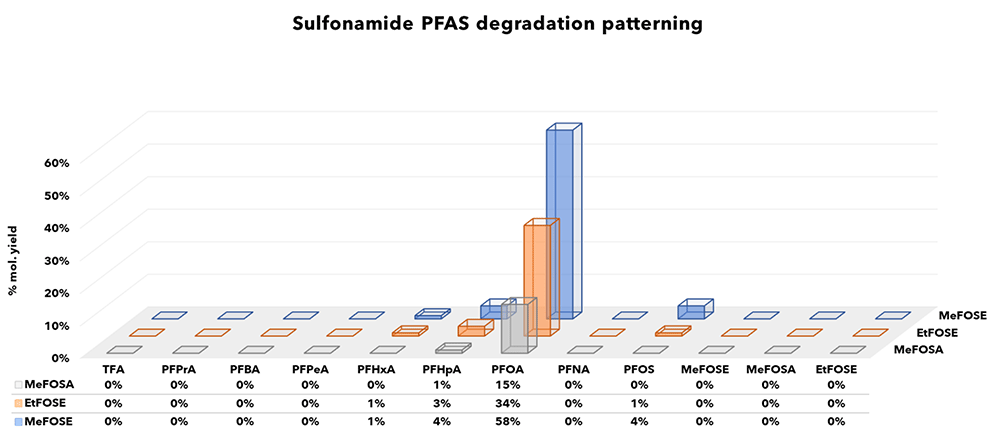
Figure 4: Sulfonamide linked PFAS products
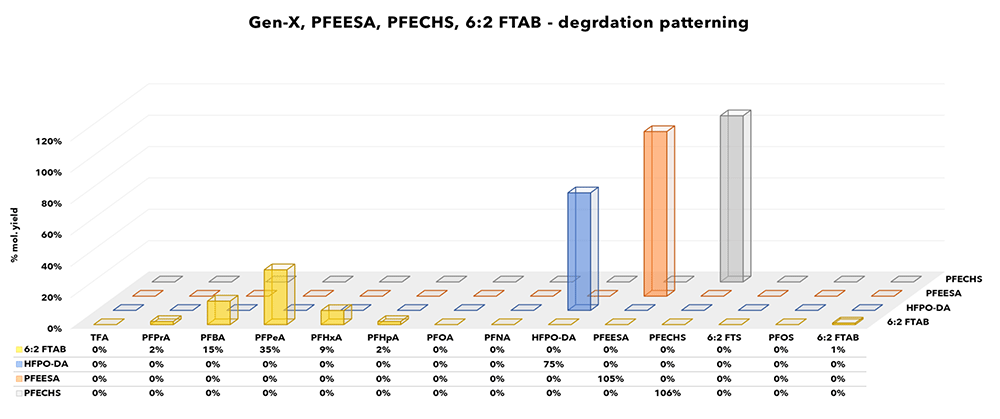
Figure 5: Gen-X, PFEESA, PFECHS, 6:2 FTAB products
Other PFAS
Replacement PFAS products including the perfluoroether Gen-X and PFEESA species did not exhibit degradation products included in traditional PFAS analytical suites and hence their presence may have been easily missed under legacy environmental monitoring projects. In contrast, polyfluoroether compounds in which complete substitution with fluorine does not occur (e.g., ADONA) have been
reported to degrade under TOP-A conditions by other researchers.1
Perfluoroethylcyclohexane sulfonate (PFECHS) belongs in the same chemical class as PFOS although can be distinguished from the better known perfluorinated sulfonic acids (PFSAs) by the presence of a cyclic perfluorinated moiety. Industrially it has been used as an erosion inhibitor in aircraft hydraulic fluid, but its inclusion in PFAS lists is relatively new. Under the experiment PFECHS did not degrade, and as for Gen-X and PFEESA, TOP-A would not have included contributions from PFECHS contamination.
6:2 FTAB has received attention recently due to its inclusion in AFFFs. Although it is a telomer based product and exhibits the characteristic distribution of PFCA products, it is distinctive because it is also a zwitterion.
ALS Australia’s TOP-A Services
Since the emergence of PFAS as contaminants of concern, chemists have battled hard to devise analytical solutions. ALS provides analytical services for many of today’s prevailing solutions including TOP-A. Total oxidisable precursor assay is a useful technique, but caution is required with data interpretation. However, despite challenges associated with TOP-A, an understanding
of degradation patterning can be helpful rather than hindering, for example, providing a way to infer manufacturing processes and hence PFAS sources (e.g. telomerisation vs ECF). Enhancements are also frequently being published on TOP-A.
ALS Australia offers services for TOP-A in various matrices, and method codes and matrices are summarised below:
| Method | Description | Water LORs (μg/L); | Solids (mg/kg) | Products (eg. AFFFs (mg/kg) |
| EP231X (TOP) | PFAS after Oxidation - Standard Level | 0.01-0.1 | 0.0002-0.001 | 0.02-0.1 |
| EP231X-LL (TOP) | PFAS after Oxidation - Low Level | 0.002-0.01 |
1 Improved Total Oxidizable Precursor Assay for Quantifying Polyfluorinated Compounds Amenable to Oxidative Conversion to Perfluoroalkyl Carboxylic Acids; Katerina Tsou, Edmund Antell, Yanghua Duan, Christopher I. Olivares, Shan Yi, Lisa Alvarez-Cohen, and David L. Sedlak. ACS ES&T Water 2023 3 (9), 2996-3003
Get in touch with us
Our technical experts and customer services teams are here to discuss your PFAS needs including elucidation of the presence of PFAS ‘dark matter’ and assist with interpretation of TOP-A results.:
Brisbane
Sydney
Melbourne
Perth





















































