enviromail 09 canada cyanide analysis maximizing data quality

All cyanide compounds contain the C≡N or cyano group, and can take many forms, including free cyanide, simple cyanides, complex metallocyanides, and organic cyanides (nitriles). The cyanide ion (CN-) causes mammalian toxicity by binding with the enzyme cytochrome C oxidase, affecting the central nervous system and heart. Human exposures to as little as 1.5 mg/kg of body weight can cause death. The acute toxicity and widespread industrial usage of cyanide makes environmental testing critical, particularly for mine tailings and effluents from industries that utilize cyanide.
Cyanide Use & Regulation
Cyanide is well known for its use in the metal mining industry, where sodium cyanide is often used for extraction of precious metals (mainly gold and silver) and some base metals. Tetrasodium ferrocyanide is frequently used in many regions of Canada as an anticaking agent in road salts and may be released to the environment by associated runoff. Cyanides may also be generated incidentally by manufacturing processes that use high temperature and pressure, such as in blast furnaces used by iron and steel manufacturing mills.
Cyanide is regulated because of potential significant impact not only to human health but also to the environment. CCME guidelines exist for free cyanide in both freshwater (aquatic life; 5 µg/L) and soil (0.9 – 8 mg/kg).
Cyanide Measurement Principles
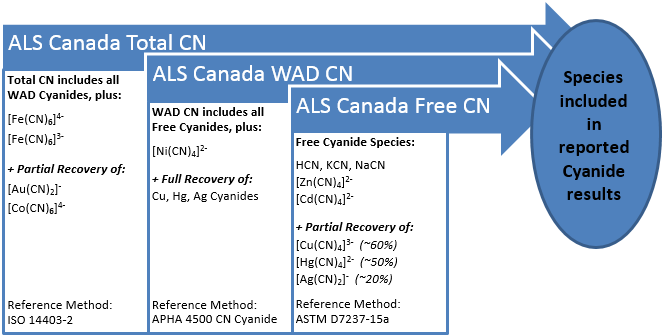
Most cyanide analysis involves the selection and use of testing methods that are designed to measure one or more of three empirical categories that encompass the various forms of cyanide compounds that may exist in a sample:
Free Cyanide – Free cyanide is the sum of HCN and CN- and cyanide bound in weak metal-cyanide complexes that are easily dissociated into free cyanide at pH 6 and room temperature (ASTM D7237-15a). Free Cyanide includes NaCN, KCN, [Zn(CN)4 ]2 -, [Cd(CN)4 ]2 -, [Cu(CN)4 ]3 - (~60% recovered), [Hg(CN)4 ]2 - (~50% recovered), and [Ag(CN)2 ]- (~20% recovered). Organic cyanides are excluded.
Weak Acid Dissociable Cyanide (WAD CN) – An operationally defined group of cyanide species that undergo dissociation and liberate free cyanide when refluxed under weakly acidic conditions (pH 4.5). WAD cyanide provides a conservative estimate of toxicity as it recovers both free cyanide and weak metal cyanide complexes (ASTM D6696-16 and APHA 4500 CN-). Weak Acid Dissociable CN includes all Free Cyanide species plus all nickel, copper, mercury, and silver cyanides. Organic cyanides are excluded.
Total Cyanide (TCN) – The sum of some organically bound cyanides, free cyanide ions, complex cyanide compounds and cyanide bound in simple metal cyanides, with the exception of the cyanide bound in gold, cobalt, platinum, ruthenium, and rhodium complexes, which are not fully recovered, and of thiocyanate (ISO 14403-2:2012).
Free cyanide is directly responsible for cyanide’s toxicity and environmental effects, but complexed cyanide species found within the WAD CN and TCN categories can liberate free cyanide with exposure to strong UV light and/or under acidic conditions.
ALS Canada Cyanide Analysis Methods
All test methods for Total and WAD cyanide species use reactions induced by some form of chemical and physical treatments to break down complex cyanides into free cyanide ions which can be detected and quantified by colourimetry or other measurement techniques. Classical wet chemical cyanide analysis procedures for these tests are highly prone to interferences due to the severe conditions imposed during manual digestion and distillation.
ALS Canada’s standardized automated cyanide analysis methods utilize Segmented Flow Analysis (SFA) to determine Total Cyanide, WAD Cyanide, and Free Cyanide in waters and soil leachates, and are now used exclusively for all environmental testing of cyanides at all our Canadian labs (excluding some waste classification testing). The ALS Canada method for Total Cyanide (ISO 14403) is particularly advantageous, since it replaces classical manual digestion/distillation (which uses extended heating with strong acids) with on-line UV irradiation to decompose strongly bound metal-cyanide complexes, causing only minimal conversion of SCN to Free Cyanide. The WAD and Free Cyanide methods liberate targeted cyanide species by adjustment of pH to 4.5 and 6.0 respectively. For all three methods, on-line membrane dialysis diverts liberated HCN gas from the sample matrix into a reagent stream for colourimetric measurement, following the same chemistry and principles as classical cyanide methodology. The significant advantages of cyanide analysis by these modern, automated and optimized methods versus classical manual techniques include:
- Improvement in data quality through reduced interferences.
- Improved precision by eliminating variation inherent in manual techniques.
- Shorter analysis times reduce turnaround times and increase sample handling capacity.
- Reduced usage of chemicals and acids (OH&S and environmental benefits).
- Less sample required (60mL), with associated benefits for sampling, waste reduction, transport cost, etc.
Management Interferences
Many sources of interferences can impact most cyanide analysis methods to varying degrees. The ALS Canada cyanide test methods are designed to minimize all common interferences to the greatest extent possible:
Thiocyanate (SCN) – SCN is a common positive interference for all Total Cyanide methods. Thiocyanate is often present (sometimes at high ppm levels) in mining effluents. SCN interference on the ALS Canada ISO 14403 TCN method averages 0.2% – 0.5% conversion to TCN (which means a 10 mg/L SCN concentration can still cause a potential false positive for TCN of 0.020 - 0.050 mg/L). Other TCN test methods (including the classical sulfuric acid distillation method) can generate far greater interferences from SCN. This highlights the importance of understanding the potential impact of SCN concentrations in your samples when testing for cyanide.
Nitrate (NO3) – High Nitrate can enhance conversion of SCN to CN- in Total Cyanide methods, but does not cause false positives on any of the ALS Canada CN methods (all CN species measure < 0.001 mg/L at 20 mg/L NO3).
Nitrite (NO2) – Although rare in environmental samples, high Nitrite can cause false positives for TCN (e.g. 5 mg/L NO2 can cause a TCN false positive of ~ 0.01 mg/L). The laboratory can treat samples to eliminate this interference if informed about high Nitrite concentrations in samples
Oxidizing agents (e.g. Chlorine, Peroxide) – Strong oxidizing agents can decompose most WAD CN species. Samples containing free chlorine or other oxidants should be treated with sodium thiosulfate or sodium borohydride in the field.
Sulfide (S2-) – High Sulfide can cause either positive or negative interferences for cyanide methods. Even at 50 mg/L, Sulfide causes no positive interferences for any of the ALS Canada CN methods (all species < 0.001 mg/L). However, under basic conditions (i.e. after NaOH preservation), Sulfide in samples can convert Free Cyanides to SCN (causing false negatives or low bias). ALS can provide a field treatment kit to remove Sulfide at time of sampling.
Ultra Violet light – Avoid exposure of samples to UV light, which can decompose many cyanide complexes to Free Cyanide. ALS Canada provides our clients with opaque (black) sample containers to eliminate this issue.
A follow-up Enviromail will describe best practice field sampling techniques to reduce or eliminate interferences from sulfide or oxidizing agents at the time of sampling.
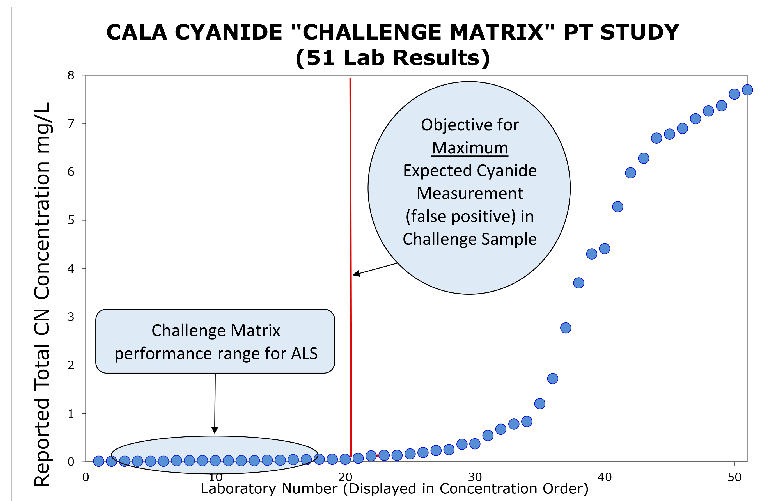
As an illustration of the effectiveness of the ALS Canada cyanide methods, we refer to a 2013 study by the Canadian Association for Laboratory Accreditation (CALA). CALA issued a blind “Challenge Matrix” Proficiency Test (PT) sample, for analysis by all CALA labs accredited for Total Cyanide analysis. The challenge sample contained high levels of known TCN interferences (particularly thiocyanate), prepared as per ASTM D7365-09.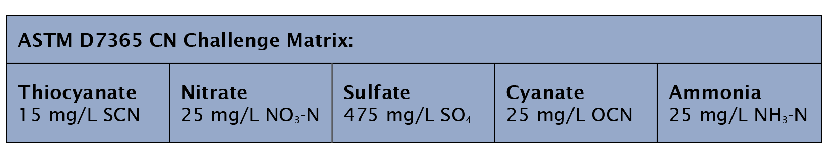

The challenge sample did not contain Cyanide. According to ISO 14403, ≤1% of Thiocyanate should be converted to CN in Total Cyanide methods, if correctly optimized. In addition to SCN, the challenge matrix includes other interferences that enhance SCN degradation. More than half of 51 Canadian laboratories accredited by CALA for TCN reported false positives that exceeded the 1% SCN conversion expectation of the study (all results to the right of the red line). All ALS Canada challenge sample results by our optimized ISO 14403 TCN method met the study objective (averaging <0.5% SCN conversion to TCN), as shown in the graphic below.

Most of the common interferences for cyanide test methods are rarely encountered in typical environmental samples (with the exception of mining effluents); however, with a good understanding of the interferences that can potentially occur with cyanide analysis, environmental practitioners can proactively avoid unexpected data quality issues, and will know when to contact the laboratory for guidance to handle complex or challenging sample types.
Limits of Reporting (LOR)
Routine reporting limit 0.005 mg/L
Low Level reporting limit 0.001 mg/L
Sampling Requirements, Holding Times and Safe Handling Levels
Opaque (black) 60mL Cyanide HDPE bottle plus sodium hydroxide preservative (target pH ≥ 12).
Samples expected to contain free chlorine or other oxidants should be treated with sodium thiosulfate or sodium borohydride (contact ALS for guidance and reagents). If test samples are expected to contain sulfide, contact ALS for a field treatment kit.
The maximum holding time for free, WAD and total cyanide analysis in preserved water samples is 14 days.
The ALS Canada safe handling concentration limit for Total Cyanide is 40 mg/L for waters, or 400 mg/kg for solid samples. Samples expected to contain greater than these safe handling limits must be identified and flagged prior to submission for any testing. To protect the safety of our staff, the maximum Total Cyanide sample concentrations that will be accepted by our labs is 400 mg/L for waters or 4,000 mg/kg for solid samples.
References
ISO 14403-2 (2012): Water Quality - Determination of Total Cyanide and Free Cyanide using Flow Analysis (FIA and CFA) – Part 2: Method using Continuous Flow Analysis (CFA).
ASTM D6696-16: Standard Guide for Understanding Cyanide Species.
APHA Method 4500 CN- O (2016): Total Cyanide and Weak Acid Dissociable Cyanide by Flow Injection Analysis, in “Standard Methods for the Examination of Water and Wastewater”.
ASTM D7237-15a: Standard Test Method for Free Cyanide and Aquatic Free Cyanide with Flow Injection Analysis (FIA) Utilizing Gas Diffusion Separation and Amperometric Detection.























































