EnviroMail 21 Canada
PAH False Positive or Bias Risk from Suspended Solids in Groundwater
The objective of environmental groundwater sampling is to collect a water sample that represents the composition of all mobile groundwater (GW) constituents in an aquifer, which may include both dissolved phase and colloidal materials that can migrate through the aquifer.

For inorganic substances such as metals or nutrients, the “dissolved fraction” is generally defined as the fraction that passes through a 0.45 µm filter. Colloids range in size from approximately 0.001 – 1 µm, so the smallest and most mobile colloids are included within this definition. Filtration is not permitted for PAHs and other organic substances with low water solubility because filter media tend to sorb and strip organics from the aqueous phase. Because of this, for regulatory compliance purposes, environmental laboratories are obligated to analyze all groundwater samples “as-received”, including any particulate matter that may be present. Collection of groundwater samples that represent mobile and dissolved phase organic parameters like PAHs is challenging, and requires expert techniques for well development and sampling to avoid artifacts from particulate matter entrained from the well during sampling.
PAH False Positives or Bias due to Entrained Solids
The most toxic PAHs are those with higher molecular weights (“heavy” PAHs), particularly benzo(a)pyrene (B(a)P). Heavy PAHs have very low water solubility and high Koc (soil adsorption co-efficient), so they tend to sorb to particulate matter, especially where high in organic carbon content. Groundwater samples contaminated with solids disturbed during sampling can trigger false positives or high bias to analytical results, if the objective is measurement of dissolved phase and mobile PAHs. PAHs are particularly susceptible to this phenomenon because they are often found in sediments and soils at low part-per-million levels, while water quality standards for some PAHs can be five or more orders of magnitude lower. Several Canadian jurisdictions have B(a)P groundwater standards as low as 10 parts-per-trillion; at such low levels, eliminating false positives due to suspended solids in groundwater samples can be challenging.
In fact, one can easily predict the potential impact of Total Suspended Solids (TSS) on groundwater PAH concentrations if the concentration of PAHs in soils surrounding a groundwater well are known.
 Groundwater Sampling
Groundwater Sampling
Approximate predicted PAH water concentrations (as µg/L) due to TSS artifacts are equal to the PAH concentration of artifact solids (mg/kg) multiplied by the TSS concentration of those solids in the groundwater sample (mg/L), divided by 1000:
Until recently, environmental laboratories frequently received groundwater samples for PAH analysis containing extremely high TSS, sometimes at percent levels, due to the challenges of collecting large volumes of water (typically 500 mL – 1 L) from monitoring wells. Fortunately, analytical testing methods for PAHs and other organics have improved dramatically in recent years, such that PAH in groundwater samples can now be tested to low part-per-trillion levels using sample sizes of 100 mL or less. This has greatly simplified the collection of artifact-free high quality groundwater samples, especially when using low flow or passive sampling.
Confirming PAH False Positive Impacts from TSS
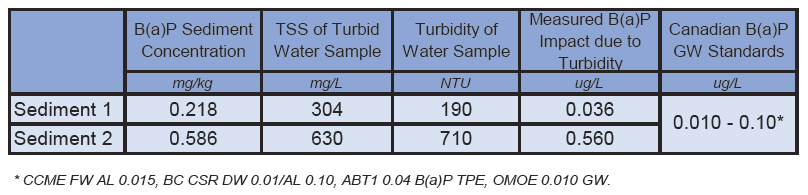
To verify and quantify the potential for false positive impacts to PAH due to entrained solids in groundwater samples, ALS created two simulated highly turbid water samples by combining lake water with small amounts of fine-grained silty sediments. These test samples were collected from two areas within an urban park, and were expected to be pristine with low or non-detectable PAH levels. The TSS content of these samples were 304 mg/L and 630 mg/L (see photo). Surprisingly, we found very significant sub part-per-billon levels of heavy PAHs in these simulated water samples due to unexpected PAH contributions from these suspended lake sediments, possibly due to the site’s proximity to roads and vehicular traffic. In fact, in both test samples, B(a)P impacts due to suspended solids exceeded some of the most commonly applied Canadian groundwater standards (CCME, BC CSR, Alberta Tier 1, and OMOE), with results as high as 0.56 µg/L (almost 60x the lowest applicable B(a)P groundwater standards!). This study demonstrated the potential to exceed PAH groundwater standards due to artifacts from suspended solids, even where no significant PAH contamination was initially suspected from associated soils or sediments.
Impact of B(a)P in Sediment on Groundwater Test Results

Suspended Solids can Cause PAH False Positives or Bias
Particulate vs. Water Fractions of Selected PAHs
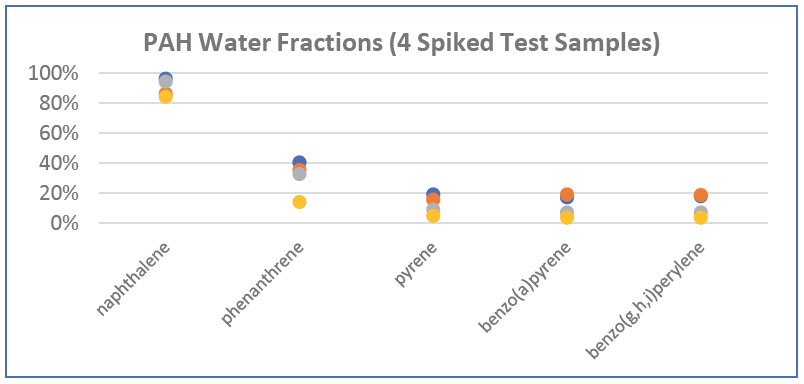
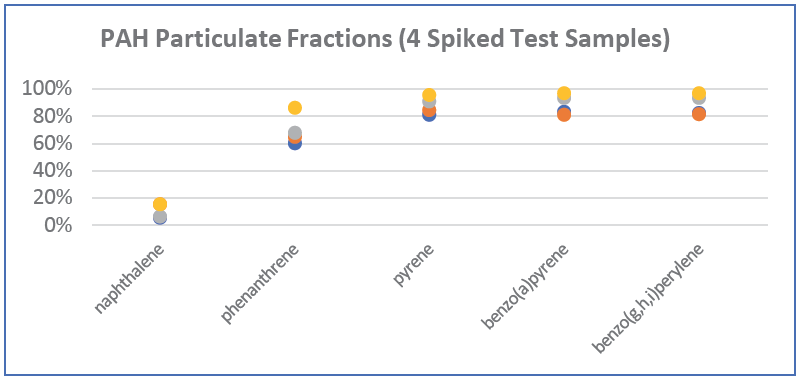
ALS also fortified (“spiked”) our turbid test samples with low concentrations of dissolved PAHs (at 0.1 and 0.5 µg/L; well below solubility limits), to assess the fraction of each PAH substance that would remain in the dissolved phase versus the fraction that would sorb to suspended solids after a ten-day equilibration period. We found that naphthalene remained 85-95% in the dissolved phase, while heavier PAHs sorbed much more strongly to the particulate fraction, with a 60-80% particulate-sorbed fraction for phenanthrene, increasing to 80-97% in the particulate fraction for the 4-, 5-, and 6-ring PAHs. Graphical illustrations of the relative particulate fractions versus water fractions that we observed for our four spiked test samples are shown in the figures below for five representative 2-6 ringed PAH substances.
Naphthalene, the lightest PAH, with only 2 rings, has a relatively high water solubility of 1,900 µg/L (@ 10° C), and tends to exist primarily in the aqueous phase. In contrast, benzo(a)pyrene is a 5-ringed PAH with very low water solubility of 1.6 µg/L (@ 25° C), with a much higher tendency to sorb to the particulate phase. The degree of sorption to the particulate phase is also a function of the Koc of each PAH and the organic carbon content of the suspended solids, with higher organic carbon content causing a higher tendency for sorption, especially for heavy PAHs.
Minimizing Solids in Groundwater Samples
In general, good sampling practice should result in clear groundwater samples with very low turbidity. Techniques to minimize turbidity and entrained solids in groundwater samples are well-documented. Groundwater wells should be constructed using recognized best practices, and must be fully developed to remove particulate matter from the aquifer close to the bore screen, and to clear any drilling mud residues. Well development requires agitation and removal of bore water until it is virtually particulate-free and of consistent quality. Low flow or passive sampling are recommended to collect groundwater samples from the screened zone of a well with the least amount of disturbance of solids. Bailers used for sampling can cause well disturbance and should be avoided if possible, especially for wells where false positives or bias due to turbidity are suspected.
Carefully control and monitor the turbidity of groundwater samples during collection. Allocate the least turbid samples for organics testing, especially for PAH and other semi-volatile organics (SVOCs) or extractable hydrocarbons; these parameters can be strongly sorbed to particulate matter, and can exhibit significant high bias due to turbidity artifacts.
Identifying PAH Artifacts from TSS
If you see unexplained variability with PAH results in groundwater wells, or if you identify groundwater PAH water concentrations that exceed or approach water solubility limits, artifacts due to turbidity and suspended solids should be considered as a possible cause. Similar issues can also occur with other SVOCs and with extractable Petroleum Hydrocarbons (PHCs, such as CCME F2-F4, BC EPH).
A recommended diagnostic technique to assess possible impacts of particulate matter on PAH or PHC groundwater concentrations is to test for correlation of sample TSS or turbidity measurements with analyte concentrations over time. For groundwaters where PAHs or PHCs are contaminants of concern, ALS recommends to include laboratory testing of samples for TSS as a surrogate measure for sample quality, and to record field turbidity measurements.
Water Sample Collection Requirements
Water samples submitted for testing of PAH and/or PHCs should be collected in 2 x 100 mL amber glass bottles provided by ALS, which are pre-charged with solid sodium bisulfate acid preservative. Preserved PAH and PHC samples have a holding time of 14 days to extraction, and should be chilled on ice to ≤ 10°C for shipment to the laboratory.