EnviroMail 146 Australia - PFAS: Internal Standards, Surrogates and Isotope Dilution
The terms internal standard, non-extracted internal standard, extracted internal standard, surrogates, and isotope dilution have led to some confusion in the industry and the discussion below aims to provide clarity on the approach ALS takes to evaluating PFAS data in Australia.

The terms internal standard, non-extracted internal standard, extracted internal standard, surrogates, and isotope dilution have led to some confusion in the industry and the discussion below aims to provide clarity on the approach ALS takes to evaluating PFAS data in Australia.
Internal standards and isotope dilution
Analytical methods for organic contaminants are commonly optimised using internal standards (ISTDs). These are added during the method either (1) before sample preparation, or (2) after sample preparation but prior to instrumental analysis. As PFAS methods are slowly becoming standardised – e.g. with USEPA methods 533, 537.1, and 1633 – different approaches to internal standardisation have emerged. Ideally, internal standards should exactly mimic the physical and chemical characteristics of each analyte. The best internal standards are 13C or deuterium isotope labelled compounds, which are exact analogues of corresponding native PFAS analytes that can be differentiated by mass spectrometry due to small differences in their molecular weights.
Recoveries of known concentrations of labelled extracted internal standards added during the analytical process can be used to adjust measurements of native PFAS up or down to account for analytical bias. Despite widespread attention, providers of internal standards, do not offer labelled analogues for all the PFAS species a laboratory may need to analyse. Synthesis and isolation of isotopically labelled standards is complicated and expensive, and sometimes a ‘closest match’ compound needs to be chosen as an ISTD instead. When this is necessary native concentrations can still be corrected, although the process is no longer termed isotope dilution and is considered less effective.
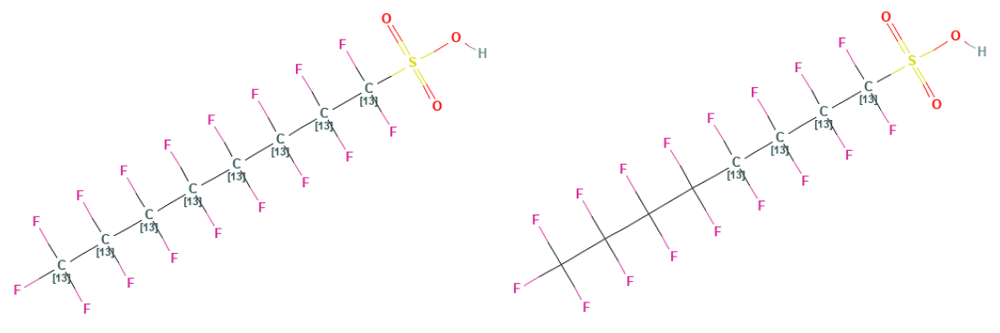
Figure 1: ALS Australia uses 13C8-PFOS as an internal standard for PFOS and 13C4-PFOS as a surrogate
Surrogates
PFAS surrogate recoveries may also be reported. Surrogates are added during sample preparation a bit like how internal standards might be but aren’t typically used to adjust target analyte concentrations. They are chosen based on chemical similarity to target analytes and are a quality control measure indicative of method performance. For example, 50% surrogate recovery could indicate that results for analytes with similar properties could also have a low bias.
Surrogates are particularly useful when a small collection of internal standards are used to quantify a large list of analytes, for example with semivolatile and volatile organic compounds. When isotope dilution is used, greater confidence is attained since each analyte is uniquely adjusted by the recovery of its labelled analogue.
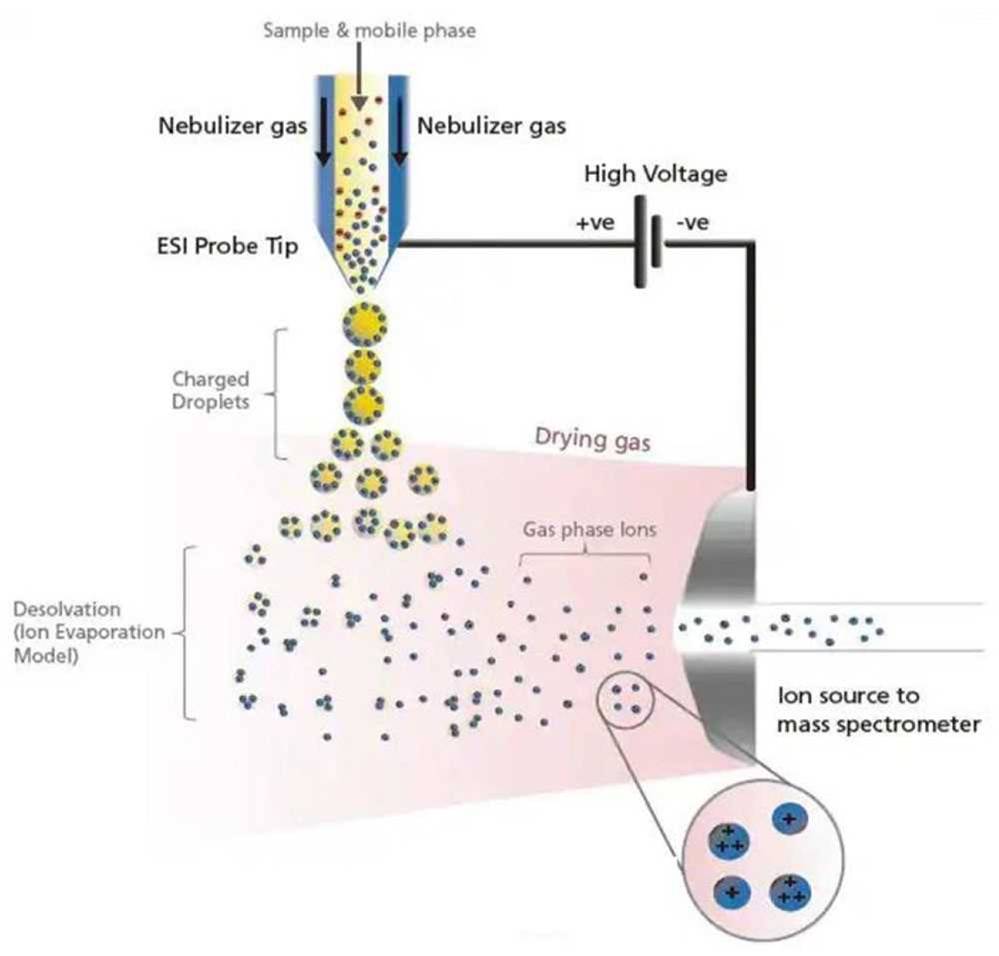
Figure 2: The electrospray ionisation process used in LCMSMS is normalised by use of matched internal standards. Image courtesy of Shimadzu Scientific
Instrumental variation and electrospray ionisation
The electrospray ionisation process inherent to PFAS analysis by LCMSMS is complex and needs to be controlled. Briefly, as sample extract elutes from the liquid chromatograph, a fine spray is formed. The droplets comprising the spray become positively or negatively charged, and since like charges repel each other, the droplets ‘explode’ as they evaporate because there becomes less surface area to support the charge. Matrix components such as salts and coextracted organics compete with PFAS analytes during this process causing enhancement or suppression. Having as many isotopically matched standards as possible normalises variability most effectively since the native PFAS and their analogues experience electrospray ionisation similarly.
USEPA method 1633
Different methods for PFAS analysis treat internal standardisation in different ways. The protocol employed by the most recent USEPA reference, Draft Method 1633, is outlined below.
For USEPA 1633 there are two types of internal standards:
Extracted internal standards (EIS)
EIS are added during sample preparation and account for variation encountered throughout the entire extraction and analysis process. Any matrix effects incurred during sample preparation are accounted for as well as those from the LCMSMS analysis.
Of the 40 PFAS species reported by method 1633, 24 have isotopically labelled analogues. The remaining 16 don’t and instead adopt the closest internal standard that behaves in a chemically similar way.
Non-extracted internal standards (NIS)
NIS are added post preparation but prior to injection and are used to report EIS recoveries as surrogates. Of the 24 EIS that can be reported as surrogates, seven compounds have a direct analogue. There are 17 EIS that don’t have a direct analogue.
Hence under method 1633 the EIS compounds serve dual purposes. Firstly, to adjust native PFAS concentrations up or down, and secondly are themselves quantified by NIS and recoveries reported as surrogates.
PFAS analysis by ALS Australia
The approach that ALS Australia takes is to dose samples with EIS at the beginning as per method 1633, leveraging EIS behaviour to adjust target analyte concentrations for sample preparation, and LCMSMS effects. The 28 compounds routinely reported by ALS’s PFAS suite have 24 internal standards (85%) for isotope dilution. ALS’s method diverges from method 1633 in its treatment of surrogates. ALS surrogates are labelled analogues of PFOS and PFOA that have the 13C in different positions (see figure 1) to the EIS making them distinguishable using mass spectrometry.
Under the 1633 regime EIS are quantified using NIS, and EIS recoveries reported as surrogates. Native PFAS concentrations are quantified using EIS accounting for variability incurred throughout the entire process. Hence the EIS surrogate recoveries are expected to be indicative of PFAS extraction efficiency but not ionisation variability.
The limited availability of NIS analogues compared to the number of EIS analogues reported by method 1633 (~29%) may also potentially limit the benefit gained by using isotopically labelled analogues. By contrast, the approach taken by ALS in Australia of adding surrogates at the beginning of the method and quantifying them using EIS also added at the beginning of the method, means that native PFAS, and surrogates undergo the same processes and surrogate recoveries are expected to reflect variability coming from both sample preparation and LCMSMS analysis. EIS abundances are still monitored and should be within 50-150% of abundances measured in matrix free analytical standards analysed on the same day. We believe that maintaining recoveries within these bounds provides sufficient confidence that variability incurred during the method remains sufficiently within control.
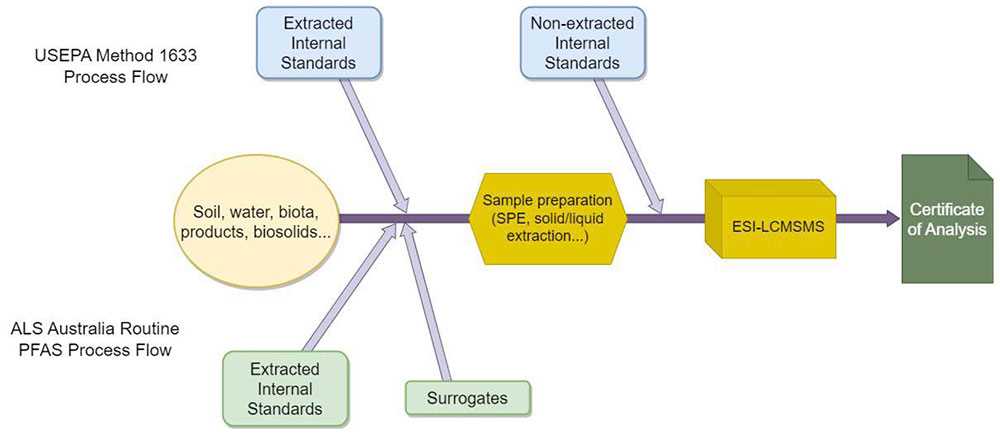
Figure 3: ALS Australia and USEPA Method 1633 Process Flow
ALS routine PFAS service offerings
ALS Australia can offer services for method USEPA 1633 via our laboratories in the United States. USEPA Method 1633 does provide high quality data, however due to its many intricacies, analysis costs and turnaround times are likely to be higher than Australian standard methods. Should your project require specific adherence to a USEPA methodology, don’t hesitate to reach out to your account manager or our customer services team for a quotation. ALS Australia’s routine service offerings are summarised below:
In addition to routine analysis for target PFAS compounds, ALS offers a broad array of supplementary techniques, including total oxidisable precursor assay (TOP-A), total organic fluorine (TOF), high resolution mass spectrometry (HRMS) for identification of novel or unknown PFAS species, and various solid leaching protocols. Additionally, should targeted analysis beyond the standard 28 PFAS suites summarised below be required, ALS is ready to support your needs.
| Method | Description | Water LORs (µg/L) | Solids (mg/kg) | Products (e.g. AFFFs) |
|---|---|---|---|---|
| EP231X | PFAS - Standard Level | 0.01-0.1 | 0.0002-0.001 | |
| EP231X-LL | PFAS - Low Level | 0.002-0.01 | ||
| EP231X-ST | PFAS - Super Trace | 0.0003-0.001 | ||
| EP231X-SUT | PFAS - Super Ultra Trace | 0.0002-0.001 | ||
| EP231X-INJ | PFAS by direct injection | 0.01-0.1 | 0.02-0.1 |
Get in touch with us
If you have any questions relating to PFAS Screening, please contact:
Brisbane
Sydney
Melbourne
Perth























































