Enviromail 25 USA - QSM 6.0 – Key Changes and Client Impact
The DoD/DOE's Quality Systems Manual (QSM) is undergoing a comprehensive update to align with ISO/IEC 17025:2017, and key changes impacting clients were discussed at the recent Joint Conference on Environmental Monitoring and Data Quality.

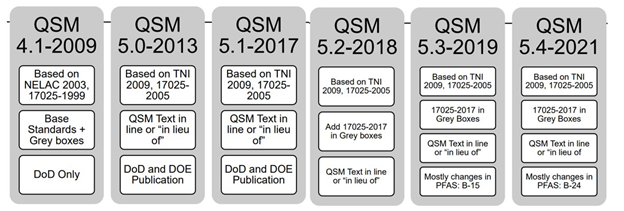
The DoD/DOE have been working to update the Quality Systems Manual (QSM) to align with ISO/IEC 17025:2017. This means the QSM is receiving a complete overhaul for structure and some requirements are changing. ALS Quality Managers recently attended the Joint Conference on Environmental Monitoring and Data Quality, where updates to the QSM were shared. This article will review those of key impact to clients. The QSM has gone through changes fairly often since 2017 (see Figure 1), but the majority of the changes have been to include and update PFAS testing requirements, and no large change to structure or content has occurred. QSM 6.0 is a complete overhaul with the following key changes.
-
The separate DoD and DOE requirements will be mostly combined. There are some DOE specific criteria, mostly around safety, which is excluded from ISO/IEC 17025:2017, that will remain.
-
The language used by ISO/IEC 17025:2017 to indicate requirement (shall), recommendation (should), permission (may), and possibility (can) will be adopted, including the use of the terms “document” and “record”. The risk-based approach incorporated first into ISO 9001:2015 and then ISO/IEC 17025:2017 will be incorporated. This allows the laboratories to focus more on outcomes instead of prescriptive requirements. It also means removal of “preventive action” language (replaced with “risks and opportunities”).
-
To eliminate influencing quality system development, guidance and examples will be removed. The DoD and DOE have come to recognize that laboratories tend to be experts in testing and there will be improved allowance for laboratories to develop their own systems and solutions.
-
When laboratories do not conduct sampling, and the Sampler neglects to include the date and time of sampling, the laboratory will no longer assume a date and time and will indicate on the report that this information was missing.
-
QSM 5.4 Appendix E content will be incorporated into Module 2 as a new section, and there will be a new Module 8 for Industrial Hygiene testing.
-
Reporting requirements (currently in QSM 5.4 Appendix A) will be moved to Module 2 Reporting Section, and the different stages for reporting will be eliminated. In QSM 6.0, all DoD/DOE reports will reflect Stage 4 requirements unless there is a contract in place with a DoD/DOE site to allow simplified reporting. There will be additional reporting criteria added for precision and bias to primarily gain understanding of uncertainty for low-level concentration when the Limit of Quantitation (LOQ) is near the project action level. Additional waiver criteria will also be added, which will require that the waiver be included in all affected data packages. The approved QAPP will not count as a waiver.
-
Appendix D will be removed primarily due to lack of use. Appendix A currently has no content (as information will be moved to Module 2) but is being held in reserve for content that is yet to be announced.
-
The Appendix B tables will be reformatted and much of the criteria will be removed when it is adequately defined in the reference methods.
Appendix C will get minor revisions, primarily regarding clarification of Method 8330 criteria and which limits are applicable when an incremental sampling method is used. -
The PT requirements in Module 1 will be updated to align better to TNI and AIHA PT requirements. There will also be guidance for analytes that are not currently included in the TNI Field of
-
Proficiency Testing (FoPT) such as 1,4-dioxane, PFAS and uranium isotopes. Criteria for which PT providers to use as a hierarchy decision tree will be defined. The preference will be to use TNI FoPT, then US or Canada based ISO/IEC 17043 PT Provider, then ISO/IEC 17043 outside Canada or non-ISO/IEC 17043, then internal PT program and finally lab determining their own precision and bias twice per year.
-
Although ISO/IEC 17025:2017 eliminated the need for a quality manual, the DoD/DOE will retain this requirement, but simplified required elements by removing the need for a quality policy statement and purchasing, complaint, and nonconforming work policies. Other policies like competence, impartiality, and consistent operation of laboratory are still required. The DoD has eliminated the need for a named Technical and Quality Manager, instead adopting the ISO/IEC 17025:2017 language for identification of personal with overall responsibility for the lab. However, the DOE will be retaining the requirements for these roles.
-
There will be a new requirement to track alterations to original versions for automated instrument outputs. This will primarily impact measuring systems that include programed integration of peaks. The DoD/DOE have noted that reanalyzing data without applying the changes does not always generate the original result and wanted better retention of original data with improved notations for why an adjustment was made.
-
All technical records will be retained for 5 years from the last use by the laboratory. There will be additional record retention updates, primarily to improve clarity on what is required to be kept.
-
QSM 6.0 will eliminate the option for hand amendments to documents and will also allow the internal audit schedule to not exceed 18 months, to promote flexibility and risk-based approaches.
-
As ISO/IEC have released a standard for reference materials (ISO/IEC 17034:2016), the DoD and DOE will eliminate the need for a second source initial calibration verification when 17034 certified reference materials are used for the calibration. There have been additional changes to the QC requirements, primarily to allow more flexibility, clarification, and alignment to reference method requirements.
During the conference, there was significant discussion around updates to the PFAS/PFOS testing programs. The DoD is currently leading a multi-laboratory validation study of Method 1633, which is expected to be completed in late 2023. As the work is largely completed, this has resulted in significant updates to the Table B-24 contents. Since most of the criteria currently in these tables will be incorporated into the EPA Method 1633, most of the language is being simplified to instead reference Method 1633 requirements. For example, the ion transitions, ion ratio, instrument sensitivity check, initial calibration verification, non-extracted internal standard compounds, bile salt standards, MS/MSD will be removed since these will be incorporated into Method 1633 and defined there. There was a very enlightening presentation provided by the DoD for common data errors observed from the DoD projects. ALS have taken note of all provided and will be doing what we can to improve our Stage 4 reports for added clarity and transparency.
Overall the conference was incredibly informative and will allow ALS Environmental to plan for these upcoming changes, which will enable the transition from QSM 5.4 to QSM 6.0 to be seamless for Clients.
Contact ALS today to learn how we can help you meet the new QSM requirements.




























