EnviroMail 127 Australia - Microbial Diversity Profiling via NGS
Bacterial diversity profiling is used for identification and classification of microorganisms present in a complex and mixed microbial community.

Bacterial diversity profiling is used for identification and classification of microorganisms present in a complex and mixed microbial community. This analysis is based on 16S rRNA gene sequencing using Next Generation Sequencing (NGS) technology and provides insight into the full microbiome.
This rapid, high-throughput and culture-free detection method has many applications in water quality management including monitoring of bacterial communities in water treatment plants, distribution systems and biofilms.
ALS offers a range of molecular tests to provide a comprehensive picture of the biological composition of the sample. Bacterial diversity profiling is part of this testing suite and is designed to estimate the relative abundance of bacteria present in the sample by sequencing seven hypervariable regions of the 16S rRNA gene. It also provides diversity estimates and functional profiling for easy comparison across temporal and spatial scales.
ALS provides bacterial diversity profiling in all water matrices (including potable, wastewaters and recycled water) and sludge/soil or sewage samples.
Guidelines
Australian water quality guidelines for fresh, marine and recycled water provides guidance on acceptable levels of various micro-organisms.
Bacterial diversity profiling via NGS is emerging as a valuable and important screening tool in water quality assessment for regulatory compliance and to rapidly and cost-effectively identify bacterial species in polymicrobial environmental samples as a routine monitoring or surveillance tool.
Method and Reporting
Method Code MM327
Reporting
Relative abundance of each organism (%), calculated using total read counts.
Diversity metrics (Shannon’s Index, True Diversity)
Relative proportion of functional groups based on bacterial identities.
Principle
The 16S rRNA gene, a ubiquitous gene in the bacterial genome, consists of both highly conserved regions and variable regions. The conserved regions serve as anchors for universal primers that allow sequencing of the variable regions that are used to discriminate between different microorganisms for identification.
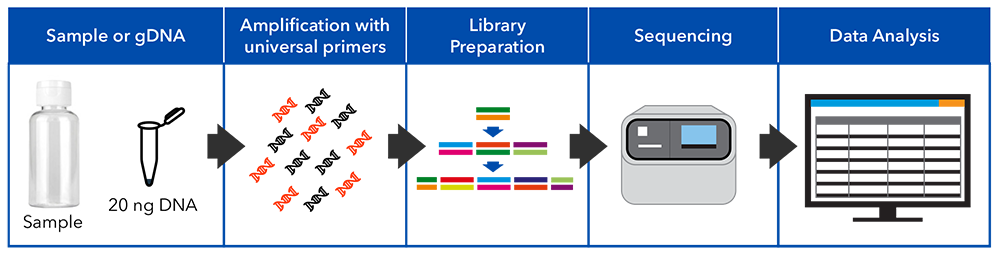
Seven of nine hypervariable regions of the 16S rRNA gene are amplified by PCR and barcoded to synthesise libraries. The barcoded libraries are pooled, templated and sequenced using an automated NGS system.
The sequences are rarefied to a standard read depth, then analysed with a 16S metagenomics analysis that aligns sequences to the reference database. Finally, the data are analysed by a proprietary ALS pipeline that provides a report summarising the bacterial community profile.
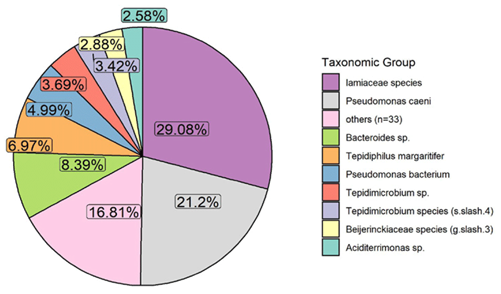
Fig. 1: Pie chart showing relative abundance of OTUs.
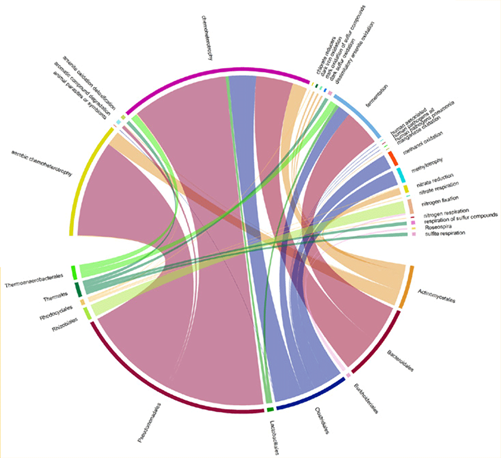
Fig. 2: Chord diagram showing relative abundance of functional groups based on taxa.
Water Industry Applications
Bacterial diversity profiling can be applied to all water, sewage, soil and sludge matrices. Extracted genomic DNA can be submitted for comprehensive examination of microbial diversity. Some of the water industry related applications are:
-
Assessment of bacterial communities within water treatment and distribution systems
-
Monitoring of bacterial re-growth and contamination in a distribution or treatment process
-
Characterisation of bacterial communities in biofilms
-
Characterisation of nitrifying bacteria in waste water treatment plants
-
Biocide monitoring of industrial processed waters
-
Drinking water surveillance for regular monitoring at strategic points.
Microbial Diversity Profiling Analysis
Identification of prokaryotic species by conventional methods requires culturing of organisms in the laboratory for hours or days. Some of these organisms, such as nitrifying bacteria, are difficult to grow in laboratory conditions. Microbial diversity profiling by NGS eliminates the need to culture organisms in the lab and can rapidly assess microbial communities in samples containing slow-growing or uncultivable bacteria.
Nine hypervariable regions (V1-V9) present in 16S rRNA genes are considered as ‘fingerprint’ regions of microbial genomes. This analysis uses sequence information from seven of these regions (V2, V3, V4, V6-7, V8, V9) to enable to identification of a broad range of bacteria to family, genus, or species level.
The test can be performed on water, sludge, or soil samples. DNA is extracted using automated extraction systems and the target regions are PCR-amplified using specific primer sets. Amplified DNA fragments are prepared as libraries for sequencing by ligation of barcoded oligo adapters. Barcoded libraries are pooled and loaded onto a semi-conductor chip and sequenced. The sequence reads are quality trimmed and rarefied before being aligned to the reference database for taxonomic assignment. These data are further analysed using a proprietary ALS pipeline to generate a report summarising the microbial diversity profile of the sample.
Results for each sample include a list of taxonomic units found in the community, ranked by relative abundance, a pie chart summarising this, a list of functional groups linked to the taxonomic units identified, ranked by relative abundance, and a chord diagram summarising this. Raw sequencing files and data are available upon request. QC samples and stringent quality checks along each step of the workflow ensure that output data meets the highest quality standards.
- This test approach is rapid, sensitive, uses small sample size and has large sample throughput capabilities.
- This analysis is a valuable tool to detect bacterial indicators in water resources and can help in better management and decision making in water quality related issues. It can also be used in agriculture, food, and health settings.
Sampling Requirements
| Holding time | 72 hours for samples stored at 5°C ± 3°C |
| Turnaround time | Standard: 20 business days (5 business days available if required) |
| Sample volume |
Low turbidity water matrix: 2 L in sterile bottles |
| Sample shipping and storage | Transport sample on ice or in a refrigeration unit |
References
Urbaniak C, Checinska Sielaff A, Frey KG, Allen JE, Singh N, Jaing C, Wheeler K, Venkateswaran K (2018) Detection of antimicrobial resistance genes associated with the International Space Station environmental surfaces. Sci. Rep. 8: 814.
Liguori K, Keenum I, Davis BC, Calarco J, Milligan E, Harwood VJ, Pruden (2022) Antimicrobial Resistance Monitoring of Water Environments: A Framework for Standardized Methods and Quality
Control. Environ. Sci. Technol. 56(13): 9149-9160.
Get in touch with us
If you have any questions relating to digital platforms for clients, please contact:
Brisbane
Sydney
Melbourne
Perth























































