EnviroMail 04 United Kingdom
1 4-Dioxane: An Emerging Contaminant of Concern
1,4-Dioxane (also known as dioxane, p-dioxane, diethylene oxide, 1,4-diethylene dioxide, and glycol ethylene ether) is a synthetic organic compound currently and historically used in various industrial applications. 1,4-Dioxane has primarily been used to stabilise 1,1,1-trichloroethane (1,1,1-TCA) and is a contaminant of concern at many chlorinated solvent release sites.
/EnviroMail-04.png?h=1200&iar=0&w=1200&hash=D1902875EE437C554AEDEDAAFFEA72A0)
1,4-Dioxane (also known as dioxane, p-dioxane, diethylene oxide, 1,4-diethylene dioxide, and glycol ethylene ether) is a synthetic organic compound currently and historically used in various industrial applications. 1,4-Dioxane has primarily been used to stabilise 1,1,1-trichloroethane (1,1,1-TCA) and is a contaminant of concern at many chlorinated solvent release sites. At these sites, it is a co-contaminant with trichloroethene (TCE) and its breakdown products.
Sampling under the United States Environmental Protection Agency (US EPA) Unregulated Contaminant Monitoring Rule (UCMR) revealed greater than 10% of U.S. drinking water supplies, representing over 90 million people in 45 states, are impacted by 1,4 dioxane. In the UK, 1,4-dioxane has been identified under the Environment Agency’s Prioritisation and Early Warning System (PEWS) as a contaminant of high risk and high certainty.
1,4-Dioxane is highly soluble in water. Given 1,4-dioxane’s mobility, a key issue in characterising impacted sites is establishing the extent of migration in groundwater. These properties have led to a concern that 1,4-dioxane plumes could potentially extend beyond that of its co-contaminants.
Uses and Potential Sources
- 1,4-Dioxane should be included in monitoring suites at all chlorinated solvent sites, particularly those where 1,1,1-TCA degradation products are present.
- Solvents used in de-greasing, electronics and many other applications may have contained 1,4-dioxane.
- 1,4-Dioxane is widespread in the UK’s ground and surface waters due to its use in personal care products and detergents.
- The US EPA identified 1,4-dioxane as a waste produced as a manufacturing by-product from rubber and plastics industrial facilities.
- Industry has used 1,4-dioxane as a component of printing inks and paints since the 1950s, either as a solvent (to help ink/paint adhere to plastic) or in conjunction with 1,1,1-TCA (as a solvent in some paints).
- 1,4-Dioxane was an additive or impurity in antifreeze and aircraft de-icing fluids so is likely to impact sites where these operations have been conducted previously.
Table 1 Physical and Chemical Properties 1,4-dioxane
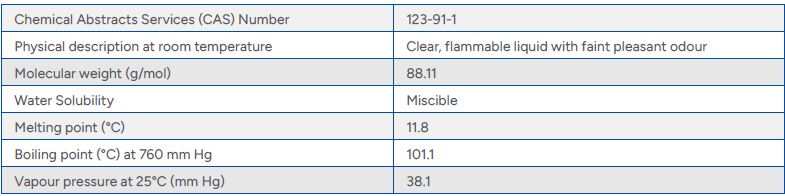
Physical and Chemical Characteristics
Those unique physical and chemical characteristics identified above, make 1,4-dioxane an increasing focus of controlled waters risk assessment. The fate and transport properties of 1,4-dioxane create challenges for investigation, assessment and remediation.
- 1,4-Dioxane is primarily a contaminant in groundwater and surface water. The miscibility and low sorption potential of 1,4-dioxane, allows it to migrate ahead of other contaminants in extended plumes.
- 1,4-Dioxane’s density is close to that of water, and it is fully miscible.
- 1,4-Dioxane has a low organic carbon partitioning coefficient (Koc), which results in low affinity for organic carbon, and is also non-ionic (e.g., uncharged). As a result, 1,4-dioxane does not bind strongly to soils or sediment and readily leaches out of soils into groundwater or surface water.
- 1,4-Dioxane’s low Henry’s law constant indicates a low potential for volatilisation in the liquid phase compared to other solvents.
- Impacts have been identified at drinking water abstractions and focus globally has be on public water supplies.
These characteristics control its partitioning in media and the relative importance of fate and transport processes.
Sampling
Sampling for 1,4-dioxane can be impacted by its polar-like characteristic, vapour pressure, boiling point, and partitioning coefficient. Because of 1,4-dioxane’s low Henry’s Law constant, following typical groundwater sampling procedures for semi-volatile organic compounds (SVOCs) is appropriate for 1,4-dioxane. No additional precautions to prevent volatilisation would be needed beyond those that would be used for other contaminants analysed by a SVOC analytical method (for example, polycyclic aromatic hydrocarbons (PAHs; e.g., benzo[a]pyrene) and polychlorinated biphenyls (PCBs)).
Passive diffusion bags (PDBs) that are not water permeable have been found to be ineffective in sampling for 1,4-dioxane. In particular, PDBs using a single low-density polyethylene (LDPE) membrane for VOCs do not diffuse the 1,4-dioxane molecule and should not be used.
Equipment used in sampling can be a source of cross-contamination. 1,4-dioxane also might be present in detergents used for de-contamination purposes. When collecting samples for 1,4-dioxane, submission of additional field and equipment blanks is recommended prior to and during sampling to ensure a robust system of QA/QC is followed.
Analytical Overview
Table 2 Sampling and Analysis Requirements
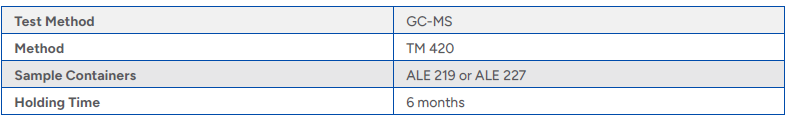
Preparation and Extraction
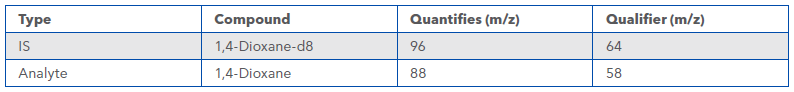
An isotope labelled analogue is added prior to sample extraction and carried through the entire extraction procedure and used as internal standard (IS) as detailed in Table 3. A microextraction is performed, using dichloromethane, to extract 1,4-dioxane from the sample.Analysis
ALS can carry out analysis of water samples for 1,4-dioxane by Gas Chromatography-Mass Spectrometry (GC-MS). The analytical approach adopted increases sensitivity using GC-MS in conjunction with isotope dilution and selective ion monitoring (SIM). The limit of detection is 0.07 μg/l.
Table 3 Quantifier and Qualifier Ions
For further information please contact:
PFAS Technical Support: pfas.hawarden@alsglobal.com
Client Services: info.ukenviro@alsglobal.com
References
- ATSDR. 2012. Toxicological Profile for 1,4 Dioxane. Agency for Toxic Substances and Disease Registry
- EA. 2023, The Prioritisation and Early Warning System (PEWS) October 2023 Tranche 1-11. Environment Agency.
- ECHA. 2023. 1,4-Dioxane. Brief Profile: European Chemicals Agency
- ITRC (Interstate Technology & Regulatory Council). 2021. 1,4-Dioxane. Washington, D.C.: Interstate Technology & Regulatory Council, 1,4-Dioxane Technical Team.
- Mohr, Thomas K. G., William H. DiGuiseppi, Janet Katherine Anderson, and James W. Hatton. 2020. Environmental Investigation and Remediation: 1,4-Dioxane and Other Solvent Stabilisers 2nd ed. Boca Raton, FL: CRC Press
- US EPA. “Method 8270: Semivolatile Organic Compounds by Gas Chromatography/Mass Spectrometry (GC/MS).”