EnviroMail 30 USA
GC-HRMS vs GC-TQ: An equivalent Technology for 1613B Dioxins and Furans
ALS has successfully implemented and validated an equivalent technology to replace the HRMS-magnetic sector instruments for testing of dioxins and furans.

Background
Polychlorinated dibenzo-p-dioxins (PCDDs or Dioxins) and polychlorinated dibenzofurans (PCDFs or Furans)are a group of compounds that are chemically similar and are considered persistent environmental pollutants (POPs). They are unintended by-products of human activities, in particular industrial and combustion processes. Dioxins and Furans are found world-wide and accumulate in the food chain, including fatty tissue of animals. Prevention and reduction of these pollutants in the environment is critical to human health and the environment in general.
Current Challenges
Method 1613B was promulgated in 40 CFR 136 in 1995 and remains the only approved method for dioxins and furans at permit levels. Current methodology for the detection and quantitation of dioxins and dibenzofurans requires use of HRMS-magnetic sector instruments. Manufacturers of these instruments are phasing out support and development of this technology. This is due to the age, shortage of parts, as well as insufficient demand as other parts of the world have adopted and transitioned to other technologies.


Figure 1: TOP: HRMS-magnetic sector Bottom: Shimadzu GC-TQ-8050
The Solution
Two ATPs (Alternate Test Procedure) were submitted to the EPA in 2022 under PAM-16130-SSI and Method 16130. Both ATPs utilize the GC-TQ technology with Multiple Reaction Monitoring (MRM) and were approved for use. It was determined that the performance of the ATP method is substantially similar to methods listed at 40 CFR Part 136 for measurement of PCDDs/PCDFs in wastewater. They are now being recommended to be included in future regulatory actions after the rulemaking process. ALS set up and validated the ATP PAM-16130-SSI method in 2022 and is currently compliant with the requirements.
Analysis and Calculation
Calibration standards are purchased from an approved vendor at the same concentrations as the current method. To test sensitivity, an additional point at the low end was used. The sample extract is injected onto a Gas Chromatograph (GC) under the same temperature and column conditions as the original HRMS method. The MS/MS will then fragment the parent compound into multiple parts to accurately distinguish the analytes. Calculation is done using a multi-point calibration and evaluated. Isotopic dilution calculation is also used to adjust target analytes based on the recovery of their associated isotopes.
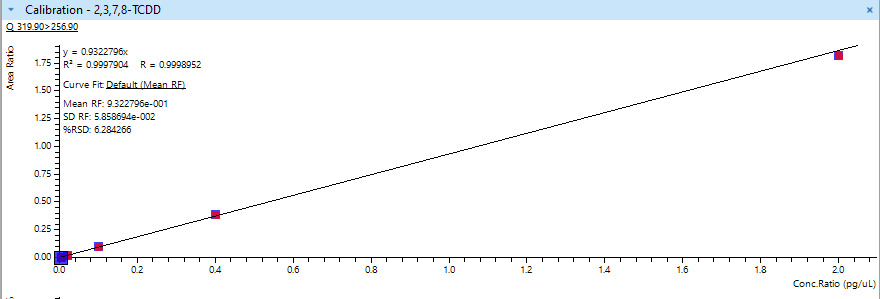
Figure 2: Calibration Curve of 2,3,7,8-TCDD, one of the most toxic of the dioxins.
Validation Data
Validation was performed in accordance with ALS defined procedures. Laboratory control studies, MDL studies, and PT (proficiency testing) were performed and evaluated. Accuracy, recovery, and precision requirements were evaluated and meet all requirements of the method. All validation data results confirm the suitability and proficiency of the ATP method in the laboratory.
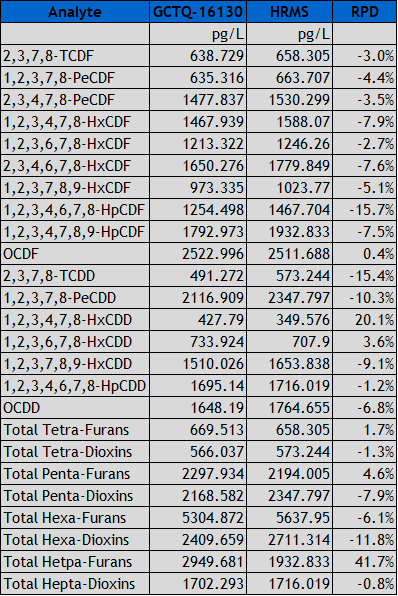
Figure 3: Comparison data of a reported PT (proficiency test) between the HRMS and GC-TQ. The same sample extract was run on both instruments.
Conclusion
The GC-TQ is a suitable technology to replace an aging HRMS fleet. Moving dioxin analysis to this technology allows for capacity increases, increased manufacturer support, and reduced downtime. This allows the laboratory to continue to provide accurate and reliable dioxin and furan results in a timely manner.
Prevent costly testing interruptions. Contact your ALS representative for more information on switching to GC-TQ.























































