Introduction to coal rank
The temperature of the earth rises with increasing depth underground. Most bituminous coals are thought to have formed between 50°C and 150°C. The geothermal gradient in sedimentary basins is approximately 25°C to 30°C for every kilometre depth. Thus, the depth of burial for bituminous coals is between 1.7 km and 6 km. As the coalification process occurs at relatively low temperatures, the coal has to remain buried at that depth for several million years. Although this seems an extensive length of time, within the geological timescale this is readily achievable..
The alteration of vegetation to coal is termed the coalification pathway. The degree of alteration of a particular coal is referred to as the coal’s rank.
In the transformation of peat to brown coal, the water saturated wood in the swamp is partly consumed by bacteria. This produces humic acids which further break down the wood material into an acid organic mush. As more material is overlaid on the pile, the force exerted on the organic material squeezes out excess water and the pore space decreases. Ultimately, more complex changes begin to occur with the release of water loving OH-groups (-OH, -COOH, -OCH etc). With increasing temperature, the organic mush is gelified and subsequently dries to a relatively solid form. At this point the organic matter has transformed to bituminous coal
In the bituminous rank range, increasing temperature results in additional water loss and initiation of the release of gases, notably carbon dioxide (CO2). Ongoing coalification into the mid-rank bituminous coals allows continued release of carbon dioxide (CO2) and increasing release of methane (CH4). Consequently, the residual material deoxygenates and dehydrogenates and the structure of the coal becomes carbon-rich. Via this process, by the time the coal transforms to anthracite is contains more than 90% carbon.
It is not only the chemistry of coal that changes with increasing rank. The structure of coal also changes. Low-rank bituminous coals contain groups of aromatic rings, crosslinked into a complex three-dimensional structure comprising carbon (C), oxygen (O), hydrogen (H), nitrogen (N), sulfur (S) and various radicals.
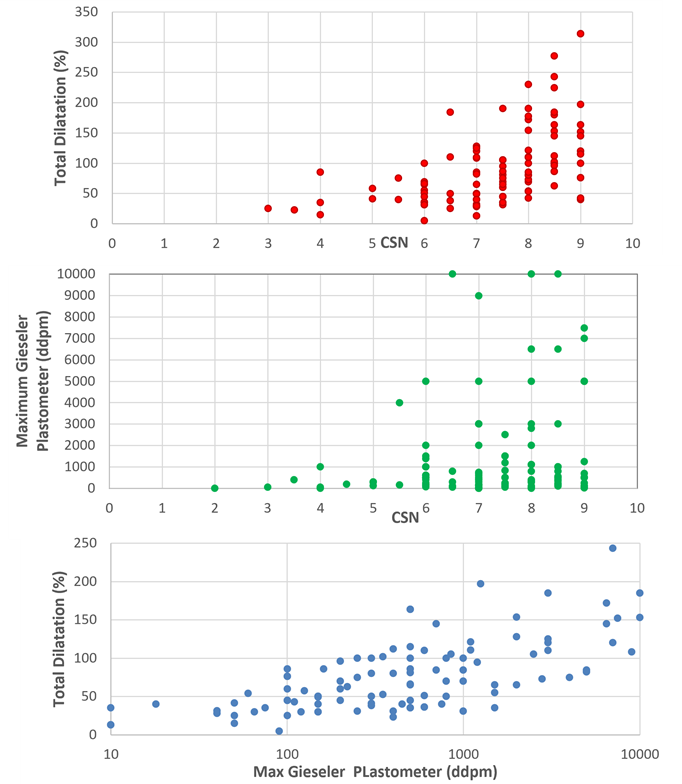
The bonds within the crosslinking structure are mostly strong co-valent bonds. However, a proportion of the C-H bonds are significantly weaker Van der Wall force linkages. The different rates at which CO2 and CH4 is driven from the coal results in an enhanced concentration of C-H Van der Wall bonds in within a sub-set of bituminous coals. It is these coals, with enhanced C-H bonds, that exhibit plastic behavior when heated in the absence of air and it is these coals that are used to make coke. Coal that are lower in rank or higher in rank compared to this subset are typically used as thermal coals in power generation. Thus, the structural changes that occur during coalification determine coal use.

Figure 1: Photograph of a Victorian brown coal specimen.

Figure 2: Photograph of Pennsylvanian anthracite specimen.
Standard tests for measuring coal rank
There are various ways to measure coal rank. The most common analyses are:
- Vitrinite Reflectance Analysis. With increasing rank, the structure of the coal becomes more graphitic and the reflectance of the coal increases
- Volatile Matter (dry, ash-free basis) from as Proximate Analysis. With increasing rank, coal has less residual volatile matter.
- Hydrogen (dry, ash -free basis) from an Ultimate Analysis. With increasing rank, coal has less residual hydrogen.
- Carbon (dry-ash-free basis) from an Ultimate Analysis. With increasing rank, coal has a higher concentration of carbon, as the other major organic components have been preferentially released.
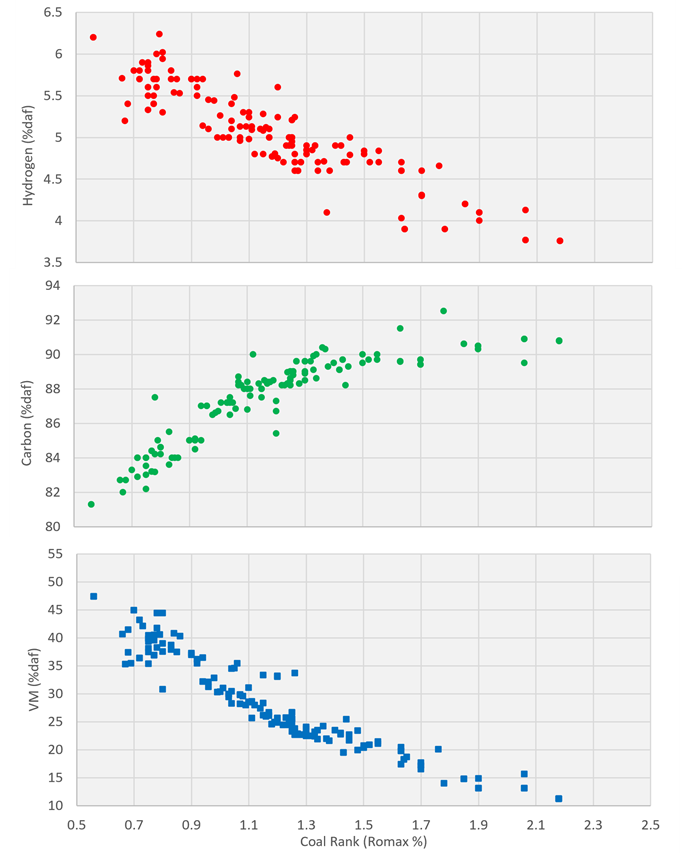
Figure 3: The relationship between various measures of coal rank including Vitrinite Reflectance, Volatile Matter, Hydrogen content and Carbon content.
 Search
Search
 English
English
 Login
Login