EnviroMail 02 Canada
Total Oxidisable Precursor Assay
There are hundreds of chemicals that can be classed as PFAS (Per and poly-fluorinated Alkyl Substances). Accredited laboratories typically determine approximately 30 of these specific chemicals. In many fire-fighting foams and other products containing PFAS, the bulk of these chemicals may be tied up in more complex molecules including polymeric compounds. Routine laboratory analysis, for the most part, includes PFAS as carboxylic and sulfonic acids and may also include one or more sulfonamides [1,2].

Total Oxidisable Precursor Assay
The Total Oxidisable Precursor Assay (TOP assay or TOPA) is a standardised pre-treatment of water samples or sample extracts (soil and water) designed to expose underlying PFAS not amenable to standard analysis.Water samples, sample extracts (soil or water) or diluted foam products are incubated with potassium persulfate (60 mM) and sodium hydroxide (0.125 M) at 85°C for 6 hours. Samples are neutralised and then run for the full suite of PFAS compounds [3,4].
Note that this is an empirical test and comparable results can only be achieved by precisely following the conditions of the test.
Under the conditions of the assay it is expected that fluortelomer sulfonates are broken down to shorter chain carboxylates by cleavage of the non-fluorinated portion of the molecule. Perfluorinated carboxylates and sulfonates are stated to remain intact under the conditions of the assay.
International Policy on the Management of Firefighting Foam
While policy is still in the formative stages in many countries globally, some countries have developed guidance to address the potential presence of unknown PFAS chemicals. Australia (Queensland Department of Environment and Heritage) recognises the potential contribution of cryptic PFAS to the environment. A policy has been developed that provides guidelines for using the TOPA for the assessment of soils, waters and foam products [5].
ALS Analysis and Reporting
ALS developed and has provided TOP pre-treatment and subsequent analysis in Australia for clients for over a year and now brings this method to Canada in support of this sector. ALS UK was also the lead laboratory working on developing the Arcadis version of the TOP Assay in 2015. ALS has also performed internal proficiency across the ALS PFAS centers of excellence in Australia, Europe, Canada and the U.S. to ensure quality and consistency remain at the highest levels for this procedure.ALS will perform the usual sample preparation used for waters (including low level waters), diluted foam products and soils followed by oxidation of the sample under the standard conditions for the TOP assay.
Please note that only the full analytical suite is available for this assay in order to account for the full range of products produced post-oxidation.
Oxidation of AFFF Products
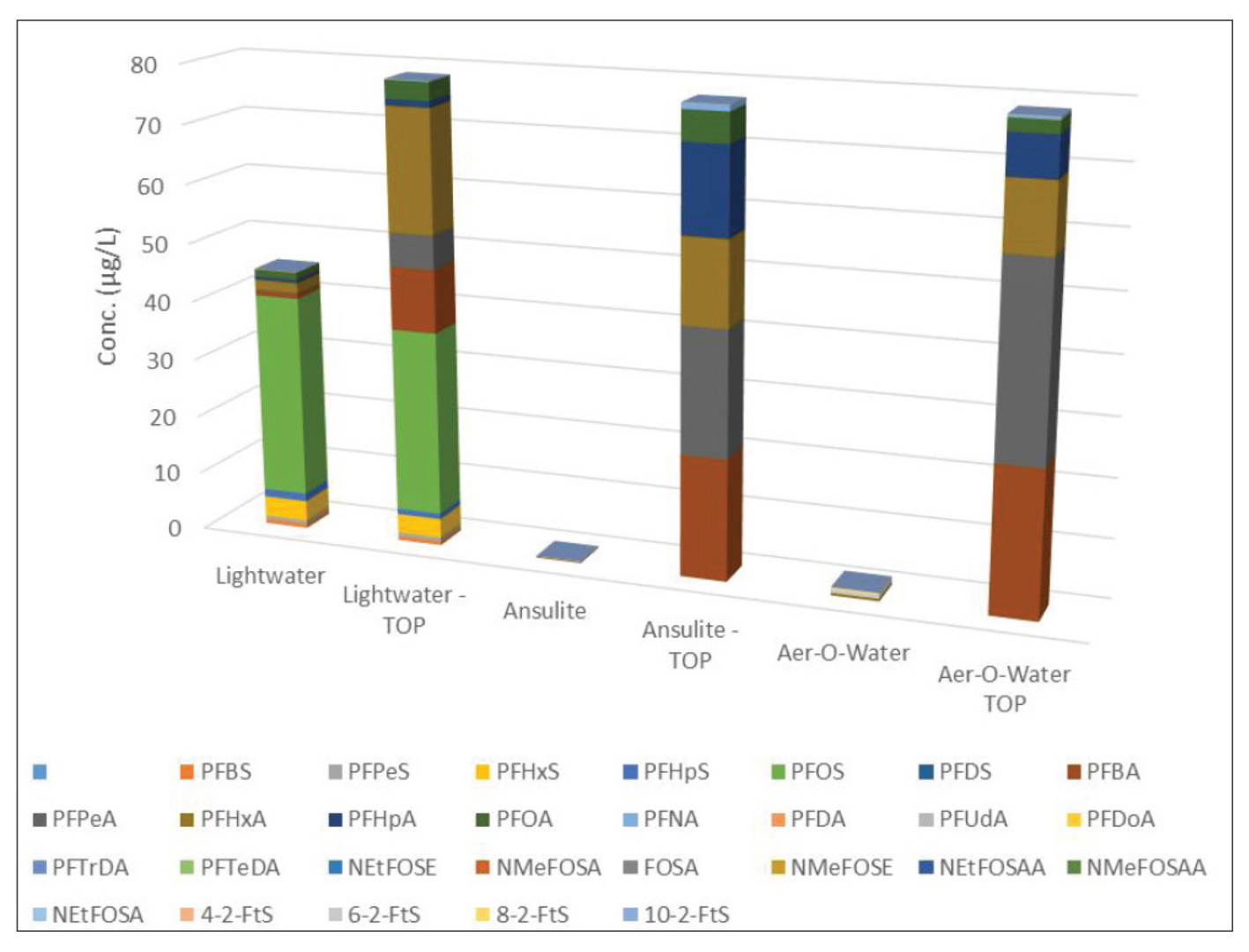
Over the last 18 months, ALS has performed numerous oxidation trials in order to obtain a deeper knowledge of the Total Oxidisable Precursor Assay. This includes the impact of concentration plus the differences between first generation and modern foams.A number of foam products were subject to the TOP alkaline persulfate digest and analysed by LCMSMS. Three products were examined: 3M light water™, Ansulite™ and Aer-O-Water™ representing the first being the classic pre-2000 AFFF and the latter two examples of more recent short-chain foams.
Figure 1 indicates significant growth in total PFAS following oxidation for Ansulite and Aer-O-Water. Growth of perfluorocarboxylic acids in 3m Light Water may be attributable to unspecified “fluoroalkyl amide derivatives” described in a 1996 MSDS from 3M in Australia. The outcomes of these digests are consistent with results reported in the literature on this subject [6].
Oxidation of Field Samples
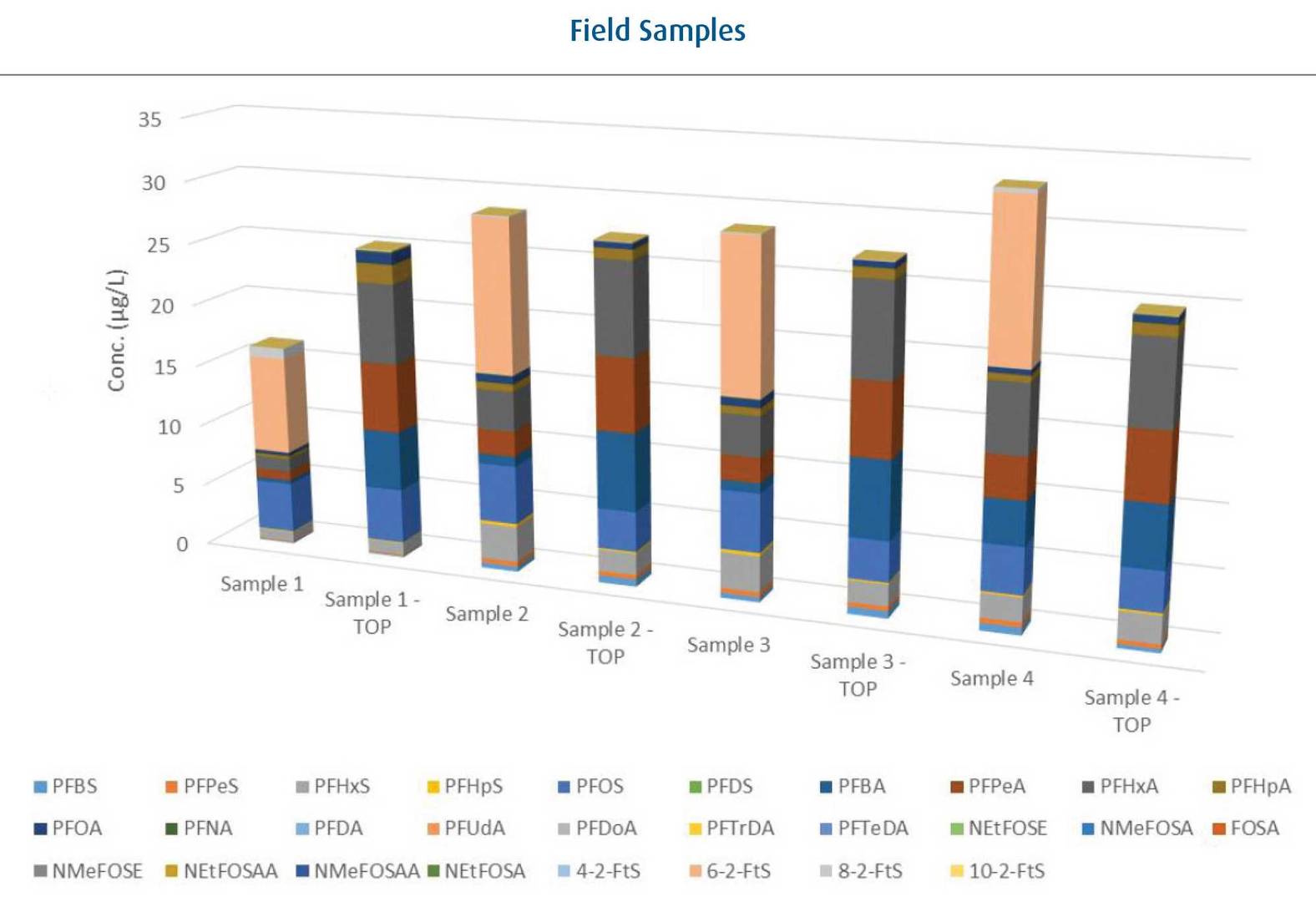
ALS test work included taking samples that recorded reasonably high results in normal testing and subjected these to the TOP assay.The pre-oxidation composition of samples indicate use of both PFOS and fluortelomer containing foams in the one location (PFOS in light blue and 6:2-FTS in pale orange).
From the composition of un-oxidised samples it can be seen that contamination originates from a mixed source – both PFOS-containing and more recent fluorotelomer foams.
Note that post-oxidation there is not much growth in total fluorinated compounds but that the fluorotelomers undergo oxidation to carboxylic acids. Also, in the case of samples 2 and 3 PFOS is seen to reduce slightly which is contrary to the assumptions behind the assay.
Discussion
The TOP assay is capable of revealing the presence of PFAS that may, given time, weather to Perfluorinated Alkyl Substances of concern but is definitely not a predictor of the endpoint of abiotic and biotic breakdown in the field.
Oxidation (and in particular, the use of activated persulfate) has been well considered as a treatment option [7]. This includes both alkaline and heat activated persulfate, both of which are used in the TOP assay. To some extent, this may explain the small loss of PFOS observed with oxidation of 3M Light Water.
In experiments performed at ALS, a 13C-labelled PFOS surrogate was added pre-oxidation and regularly recovered around 80%. Oxidation of a full analytical standard (not under standard conditions) also yielded less than a mass balance when summed, indicating some loss to shorter chain PFAS carboxylates not normally quantified (e.g. trifluoroacetic and pentafluoropropanoic acids).
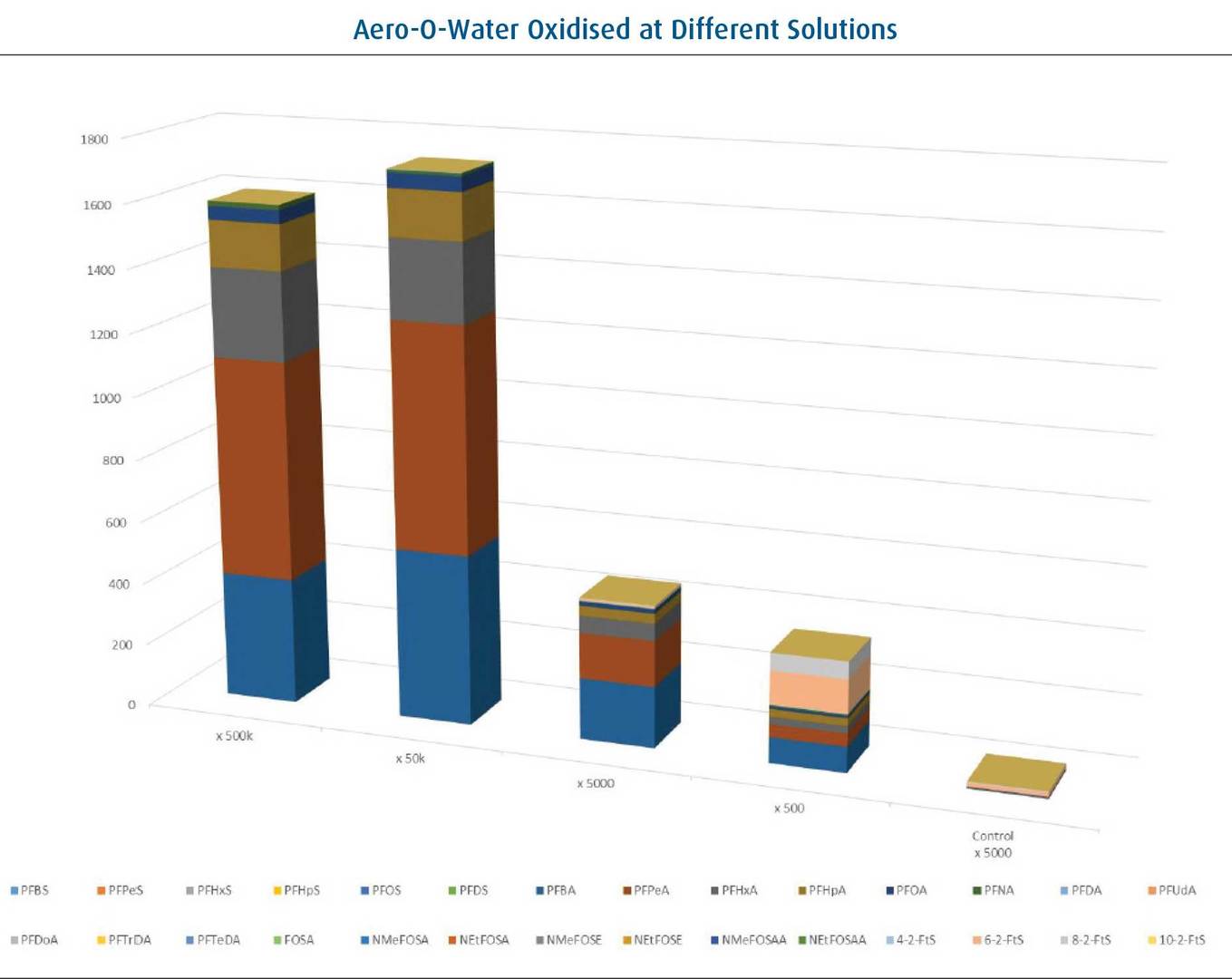
On the flip side, if the oxidant is exhausted either by competition from non-PFAS organic carbon or high concentrations of PFAS, both qualitative and quantitative conversion of AFFF PFAS precursors may be incomplete. Referring to figure 2 and the 500-fold dilution of Aer-O-Water, we see a massive growth in 6:2-FTS which is not present at lower concentrations. ALS has demonstrated that, in the absence of persulfate, incubation of the sample under the conditions specified in the assay (alkalinity, temperature and time) there is still a significant growth of the fluorotelomers. This is consistent with alkaline hydrolysis of fluorotelomer sulfonamide precursors which will yield the fluorotelomer sulfonic acid and an amine. See reference [1] discussion on National Foam (Aer-O-Water) AFFF composition.
When comparing data between laboratories, it is essential that the oxidation conditions are absolutely standardised and that sample dilutions are reasonably equivalent.
In conclusion, the TOP assay is a useful tool in exposing the potential for ongoing contamination by PFAS compounds through biotic and abiotic weathering processes. Results, however should be treated with caution, especially where a health or ecological risk assessment is required.
There may also be a case to expand analytical suites to cover other PFAS that may arise from weathering that might include some oxidation and hydrolysis and, ideally, to have better models for predicting environmental endpoints of AFFF degradation.
Each of the ALS Locations that test for PFAS (six globally) report the same list of parameters - currently a 28 compound list. Due to the standardized practices and the prescriptive nature of the TOP pre-treatment, each ALS location will provide comparable data to that presented in this study. ALS Canada has developed this expertise and is pleased to provide the 28 compound PFAS list along side the TOP assay designed to uncover potentially hidden PFAS chemicals.
References
1. Place, Benjamin J.; Field, Jennifer A. (2012): Identification of Novel Fluorochemicals in Aqueous Film-Forming FoamsUsed by the US Military. In Environmental Science & Technology 46 (13), pp. 7120–7127. DOI: 10.1021/es301465n.
2. Backe, Will J.; Day, Thomas C.; Field, Jennifer A. (2013): Zwitterionic, Cationic, and Anionic Fluorinated Chemicals in
Aqueous Film Forming Foam Formulations and Groundwater from U.S. Military Bases by Nonaqueous Large-
Volume Injection HPLC-MS/MS. In Environmental Science Technology 47 (10), pp. 5226–5234. DOI: 10.1021/es3034999.
3. Houtz, Erika F. (2013): Oxidative Measurement of Perfluoroalkyl Acid Precursors: Implications for urban runoff
management and remediation of AFFF-contaminated groundwater and soil. Doctor of Philosophy. University of
California. Berkeley.
4. Houtz, Erika F.; Sedlak, David L. (2012): Oxidative conversion as a means of detecting precursors to perfluoroalkyl
acids in urban runoff. In Environmental Science & Technology 46 (17), pp. 9342–9349. DOI: 10.1021/es302274g.
5. Queensland Government Department of Environment and Heritage Protection (2016): Operational Policy –
Environmental Management of Firefighting Foam, pp. 1–16.
6. Houtz, Erika F.; Higgins, Christopher P.; Field, Jennifer A.; Sedlak, David L. (2013): Persistence of Perfluoroalkyl Acid
Precursors in AFFF-Impacted Groundwater and Soil. In Environmental Science & Technology 47 (15), pp. 8187–8195.
DOI: 10.1021/es4018877.
7. FMC Environmental Solutions: Activated Persulfate Oxidation as a Remediation Technology for PFOS PFOA. Available
online: http://www.peroxychem.com/media/22910/FMC_Peroxygen_Talk_2011-12_Treatment_of_PFOS_and_
PFOA.pdf, checked on 8/15/2016.
8. Yang, Shewei; Cheng, Jianhua; Sun, Jian; Hu, Yongyou; Liang, Xiaoyan (2013): Defluorination of Aqueous
Perfluorooctanesulfonate by Activated Persulfate oxidation. In PloS One 8 (10), e74877. DOI: 10.1371/journal.
pone.0074877.
















































