EnviroMail 20 Canada
Non-Lethal Biopsy Sampling for Metals to Monitor and Protect Fish Populations
Each year, traditional environmental monitoring programs in Canada require the sacrifice of many thousands of fish that are killed and filleted prior to chemical analysis of muscle tissue for metallic contaminants.

Non-lethal sampling techniques for fish monitoring programs have become more and more prevalent over the past 15 years, due largely to exceptional work done by early pioneers and promoters of this practice like Randy Baker, whose ground-breaking 2004 paper on the subject1 promoted awareness and widespread acceptance of these techniques among Canadian environmental practitioners.
ALS Canada now has more than a decade of routine testing experience with the analysis of trace metals and mercury from fish biopsy samples, which typically comprise only 50-150 mg of muscle tissue. Currently, more than 15% of fish tissue samples tested by ALS Canada for metals or mercury are conducted using biopsy samples, with the proportion increasing each year. Biopsy samples are particularly well-suited for remote or international monitoring programs, because samples can be easily frozen, packaged, and shipped by air to the laboratory.

Advantages of Biopsy Sampling Techniques
The advantages of non-lethal sampling for fish tissue analysis are many. First and foremost, the health of fish populations and the ecosystems they represent can be monitored without the need to sacrifice and kill any of the fish we are aiming to protect. This is particularly important for threatened, endangered, or low-density fish populations, where traditional destructive monitoring programs may not even be permitted. Non-lethal techniques also support collection of larger numbers of test samples to achieve more representative samplings, and provide opportunities for successive samplings of the same fish over time to study temporal trends.
Non-lethal sampling is also beneficial to practitioners because it simplifies logistics and minimizes field time, while also substantially reducing sample transportation logistics and costs, since the weight and volume of samples sent to the lab are typically reduced by more than 99%.
Several studies have demonstrated excellent consistency or correlation between non-lethal biopsy test results and traditional fillet sampling techniques for many metals1,2,3, although differences may exist between some fish species and/or specific metals. Several studies have confirmed very low fish mortality rates with no sub-lethal effects following nonlethal sampling techniques1.
Non-Lethal Sampling Protocols
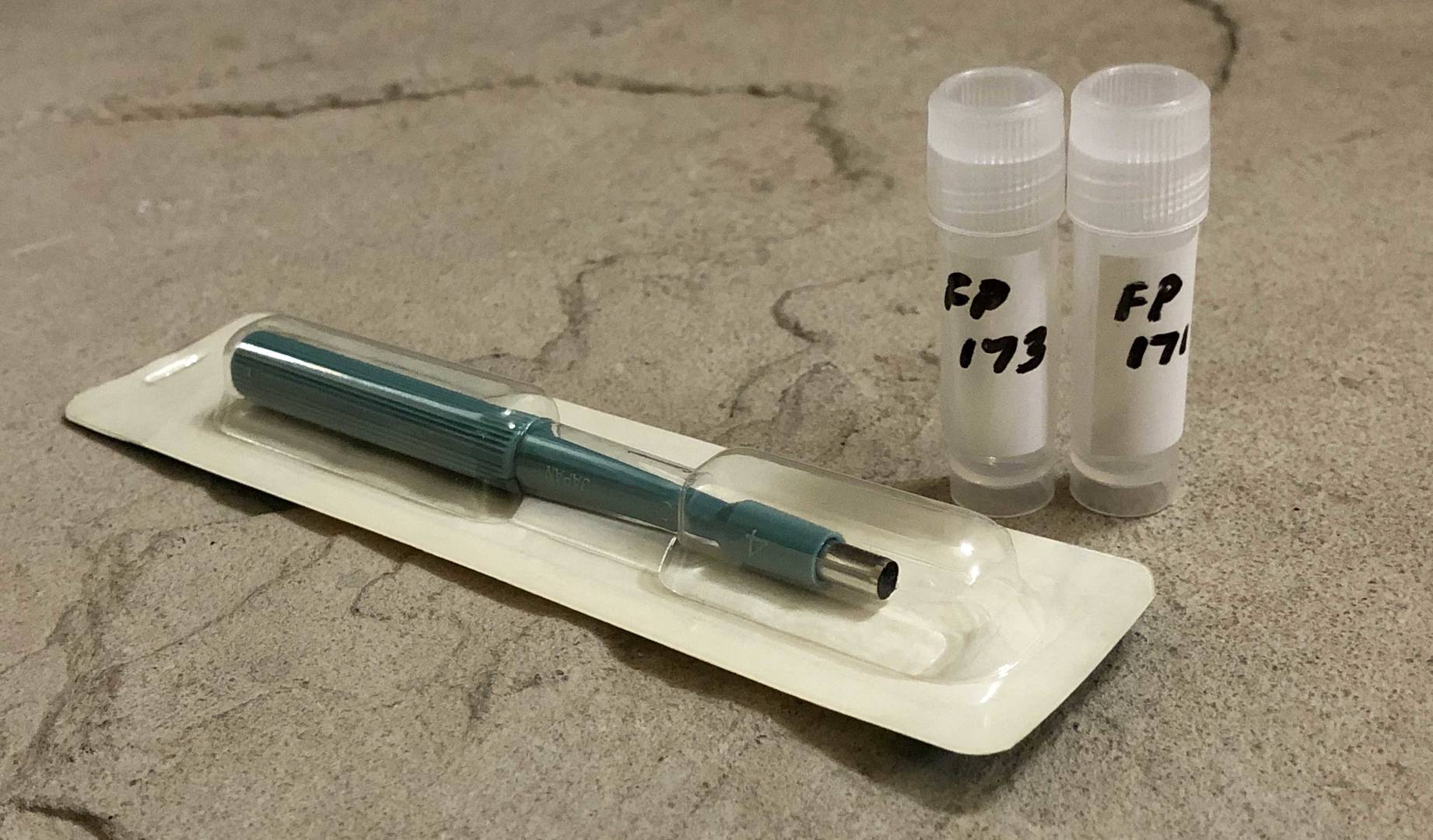
Prior to non-lethal tissue sampling, fish are caught by non-harmful techniques such as angling, short-set gill nets, or electrofishing. Fish are anaesthetized in a holding container, using tricaine mesylate (MS222), clove oil, or another suitable anaesthetic. Sterile single-use biopsy needles (e.g. Tru-Cut) or small (4-6 mm) dermal punches may be used to safely sample muscle tissue plugs from fish as small as 200 mm in fork length, and are suitable for most lakefish.
6 mm dermal punches are popular and easy to use, producing sample plugs of approximately 100-150 mg after removal of epidermis. Larger punches are available and may be more suitable for larger fish species. Biopsy needles generate smaller samples of approximately 25 mg, and have higher unit costs than dermal punches.
If required, two plugs may be collected from adjacent locations to ensure a total sample size of ≥ 50 mg. When using a dermal punch, a sterile super-glue fish bandaging material is highly recommended
(e.g. 3M Vetbond, Nexaband) to seal the wound and prevent infection. Fish are then acclimatized in a secondary holding tank prior to release. Refer to Baker 20041 for detailed recommended sampling protocols.

Sample Collection and Shipment
ALS provides convenient 3 mL polypropylene containers suitable for collection of biopsy samples (minimum recommended wet weight is 50 mg). Samples should be chilled on ice immediately after sampling, and frozen (≤ -18°C) as soon as possible (at least within 48 hours). For remote or international shipments, please contact ALS Vancouver Client Services for guidance regarding packaging and international import permits and regulations (if applicable).
Metals Testing and Results
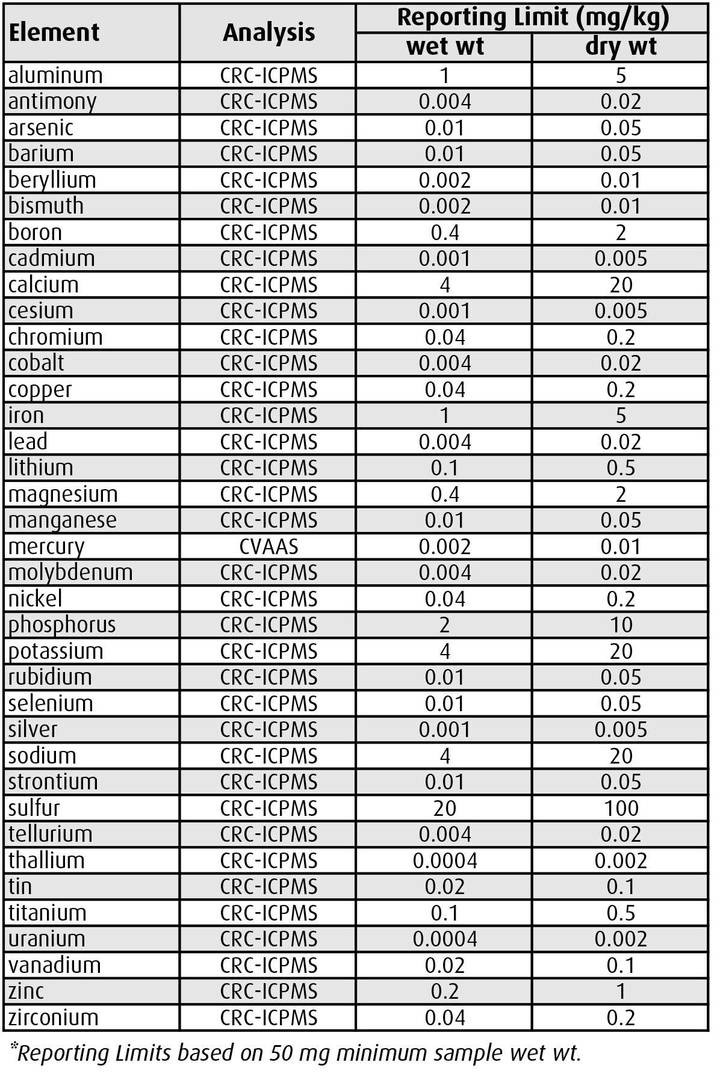
ALS offers trace level testing services for all routinely analyzed metals and mercury in biopsy samples, with Limits of Reporting as indicated in Table 1.
Samples smaller than 50 mg can be accommodated but reporting limits may increase proportionately. ALS recommends the use of dry weight test results for biopsy samples, because very small tissue samples can lose a significant weight proportion prior to analysis due to evaporation (unless wet weights are accurately measured in the field). If wet weight results are required, ALS recommends conversions from dry weight results using average moisture content for each specific fish species and tissue type.
ALS utilizes the same analytical techniques for biopsy samples as for larger fish tissue samples. Tissue digestion is conducted using a mixture of nitric and hydrochloric acids with hydrogen peroxide oxidant, as per the prescribed BC ENV Lab Manual method, with analysis conducted by Collision/Reaction Cell ICPMS or by CVAAS for trace level mercury. ALS Canada offers ISO 17025 accredited trace metals testing of biopsy samples from our Vancouver laboratory location for all parameters listed in Table 1. Refer to our CALA Scope of Accreditation for current accreditation details.
References
- R.F. Baker, P.J. Blanchfield, M.J. Paterson, R.J. Flett & L. Wesson,
2004, Evaluation of Nonlethal Methods for the Analysis of Mercury
in Fish Tissue, Transactions of the American Fisheries Society,
133:3, 568-576. - Scrimgeour, G.J., Anderson, J., Suitor, M., Wilcockson, J., Palace, V.,
2015, Development and application of non-lethal and minimally
invasive methods to quantify elements in adult Arctic Grayling and
Bull Trout in the South Nahanni Watershed, Northwest Territories.
Parks Canada Agency, Technical report, Office of the Chief
Ecosystem Scientist, Calgary, AB, Canada. - Non-Lethal Tissue Sampling of Middle Fraser River White Sturgeon
(Acipenser Transmontanus), March 2017, Environmental Protection
Division, Regional Operations Branch, BC Ministry of Environment.





















































