EnviroMail 03 United Kingdom
New Analytical Method for testing Fluorotelomer Alcohols in water
Fluorotelomer alcohols (FTOHs) are one of the major classes of per- and polyfluoroalkyl substances (PFAS). They are also one of the most well-known precursors of perfluorocarboxylic acids (PFCAs) including perfluorooctanoic acid (PFOA) and perfluorohexanoic acid (PFHxA).
.jpeg?h=2302&iar=0&w=3475&hash=D593524140D398854E6475F076F72828)
Fluorotelomer alcohols (FTOHs) are one of the major classes of per- and polyfluoroalkyl substances (PFAS). They are also one of the most well-known precursors of perfluorocarboxylic acids (PFCAs) including perfluorooctanoic acid (PFOA) and perfluorohexanoic acid (PFHxA). Their presence in surface water, groundwater and drinking water supplies represents a potential risk to human health and the environment. The ALS R&D team has recently validated a sensitive, robust and selective analytical method (pending UKAS accreditation) to quantify FTOHs using gas chromatography-triple quadrupole tandem mass spectrometry (GC-MS/MS).
Introduction
The widespread application of fluorotelomer-based substances has resulted in the extensive occurrence of FTOHs in the environment. Recent studies have focused on FTOH sources, fate, transport, and distribution in environmental media, exposure, and human health risks (references provided below).
Use of FTOHs
FTOHs are used in the synthesis of various surfactants and as intermediates in the manufacture of a variety of products with a wide range of applications including textiles, polymers, paints, adhesives, waxes and cleaning agents. FTOHs act as surfactants, lubricants and intermediate products in the manufacturing processes and can be emitted into the atmosphere during the production of fluoropolymers. Due to their high volatility, FTOHs can also undergo long-range environmental transport. Landfill leachate (Titaley et al., 2023) and wastewater treatment works are potential sources of FTOHs (Wang et al., 2020). FTOHs are a constituent in aqueous film-forming foams (AFFF) formulations and are a byproduct in fluorotelomer-based AFFF. 8:2 FTOH concentrations in AFFF ranged from 8 to 26.5 mg/L (Favreau, 2017). The detection of FTOHs at AFFF-impacted sites is therefore likely to increase as analytical methods improve.
Fate and Transport
FTOHs have been found ubiquitous in water (Ayala-Cabrera et al., 2020; Dimzon et al., 2017). Studies have also shown that FTOHs can breakdown into other persistent, bioaccumulative PFCAs in water by various biotransformation mechanisms (Dinglasan et al., 2004; Ellis et al., 2004; Wang et al., 2009; Yu et al., 2018; Zhao et al., 2013). FTOHs could therefore be considered an indirect source of PFCAs in the environment.
Exposure
Being a major precursor of common PFCAs, FTOHs may cause similar adverse health effects to human health and the environment. Human exposure to FTOH mainly occurs through ingestion pathways such as diet and drinking water (Bach et al., 2016). Because they are widely used, FTOHs have been found in various types of water sources including drinking water (Ayala-Cabrera et al., 2020; Bach et al., 2016), wastewaters (Dimzon et al., 2017; Ma et al., 2022), industrial wastewater influents and effluents (Ayala-Cabrera et al., 2020; Dauchy et al., 2017; Ma et al., 2022), surface water (Bach et al., 2016; Portolés et al., 2015), and rainwater (Kongpran et al., 2014; Mahmoud et al., 2009).
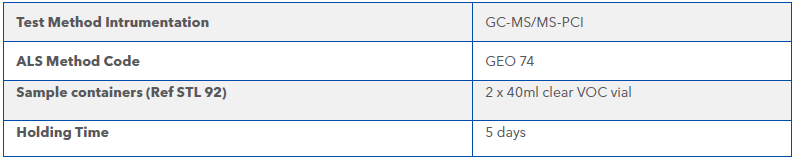
Sampling Requirements
Samples should be collected 40 mL volatile vials containing 2 mL of methanol. Vials should not be over-filled spilling methanol but with Zero headspace achieved, when collecting the sample. Samples should be returned to the laboratory as soon as possible due to the short holding times.
Table 1 Sampling and Analysis Requirements
Lab Analysis
The use of GC-MS/MS with positive chemical ionisation (PCI) improves sensitivity, selectivity, and reliability of determining FTOHs and provides detection limits as per Table 2.Table 2 Summary of Reporting
For further information please contact:
PFAS Technical Support: pfas.hawarden@alsglobal.com
Client Services: info.ukenviro@alsglobal.com
References
- Ayala-Cabrera J.F., Contreras L., Moyano E., Santos F.J. (2020) A novel methodology for the determination of neutral perfluoroalkyl and polyfluoroalkyl substances in water by gas chromatography-atmospheric pressure photoionisation-high resolution mass spectrometry. Anal. Chim. Acta DOI: 10.1016/j.aca.2019.12.004.
- Dauchy, X. Bioteux V., Back C., Colin A., Hemard J., Rosin C., Munox J., (2017) Mass flows and fate of per- and polyfluoroalkyl substances (PFASs) in the wastewater treatment plant of a fluorochemical manufacturing facility Sci. Total Environ. 576 549-558.
- Dimzon I.K., Wsterveld J., Gremmel C., Fromel T., Knepper T.P., de Voogt P. (2017) Sampling and simultaneous determination of volatile per- and polyfluoroalkyl substances in wastewater treatment plant air and water Anal Bioanal Chem 409: 1395-1404.
- Favreau, P.; Poncioni-Rothlisberger, C.; Place, B. J.; Bouchex- Bellomie, H.; Weber, A.; Tremp, J.; Field, J. A.; Kohler, M. Multianalyte Profiling of Per- and Polyfluoroalkyl Substances (PFASs) in Liquid Commercial Products. Chemosphere 2017, 171, 491−501.
- Higgins, C.; Field, J.; Deeb, R.; Conder, J. FAQs Regarding PFASs Associated with AFFF Use at U.S. Military Sites; Environmental Security Technology Certification Program Alexandria United States, 2017.
- Herzke, D.; Olsson, E.; Posner, S. Perfluoroalkyl and Polyfluoroalkyl Substances (PFASs) in Consumer Products in Norway − A Pilot Study. Chemosphere 2012, 88, 980−987.
- L.A. D’Agostino, S.A. Mabury. Aerobic biodegradation of 2 fluorotelomer sulfonamide-based aqueous film-forming foam components produces perfluoroalkyl carboxylates. Environ. Toxicol. Chem., 36 (2017), pp. 2012-2021.
- Kim, M. H.; Wang, N.; McDonald, T.; Chu, K.-H. Biodefluorination and Biotransformation of Fluorotelomer Alcohols by Two Alkane-Degrading Pseudomonas Strains. Biotechnol. Bioeng. 2012, 109, 3041−3048.
- Ma H., Peng H., Chen H., Shang W., Zheng X., Yang M., Zhang Y., (2022) Long-term trends of fluorotelomer alcohols in a wastewater treatment plant impacted by textile manufacturing industry, Chemosphere, Volume 299.
- Portolés T., Rosales L.E., Sancho J.V., Santos J., Moyano E., (2015) Gas chromatography–tandem mass spectrometry with atmospheric pressure chemical ionization for fluorotelomer alcohols and perfluorinated sulfonamides determination, Journal of Chromatography A, Volume 1413, 2015, 107-116,
- Titaley I.A., Florentino B., Cruz D., Barlaz M., Field J.A. (2023) Neutral Per- and Polyfluoroalkyl Substances in In-situ Landfill Gas by Thermal Desorption-Gas Chromatography-Mass Spectrometry Environ. Sci. Technol. Lett. 2023, 10, 3, 214–22..
- Wang, N.; Szostek, B.; Buck, R. C.; Folsom, P. W.; Sulecki, L. M.; Capka, V.; Berti, W. R.; Gannon, J. T. Fluorotelomer Alcohol Biodegradation Direct Evidence That Perfluorinated Carbon Chains Breakdown. Environ. Sci. Technol. 2005, 39, 7516−7528.
- Yan P.F., Dong S, Manz K.E., Liu C., Woodcock M.J., Mezzari M.P., Abriola L.M., Pennell K.D., Cápiro N.L. Biotransformation of 8:2 Fluorotelomer Alcohol in Soil from Aqueous Film-Forming Foams (AFFFs)-Impacted Sites under Nitrate-, Sulfate-, and Iron-Reducing Conditions. Environ Sci Technol. 2022 Oct 4;56(19):13728-13739. doi: 10.1021/acs.est.2c03669.